Mini-invasive distal pancreatectomy: a feasible and cost-effective technique
Abstract
Aim: Laparoscopic pancreatic surgery is a minimally invasive technique that has been widely applied only in the past decade. The purpose of this study was to evaluate its safety and assess whether laparoscopic distal pancreatectomy (LDP) is cost-effective compared with open distal pancreatectomy (ODP).
Methods: The medical records of patients treated for left-sided pancreatic lesions were retrospectively analysed, and the analysis of costs for hospital stay, operative time, and equipment were analysed. Twelve patients underwent LDP, while 12 patients underwent ODP.
Results: The two groups were homogeneous according to age, ASA score, BMI, and distribution of pathological findings. Both the size of the specimen (5.33 ± 3.2 vs. 5.58 ± 2.57 cm) and the number of removed lymph nodes (10.5 ± 4.3 vs. 12.1 ± 3.1) did not differ. Although LDP required a longer operative time (197.5 ± 33.7 vs. 122.5 ± 35.4 min), intraoperative bleeding, postoperative pain intensity (measured by VAS scale) and hospital stay were significantly reduced.
Conclusion: The mini-invasive approach offers several advantages compared with open surgery, including a significant reduction of blood loss and postoperative pain, and an earlier recovery. The global costs of laparoscopic surgery should be carefully re-evaluated, considering the saving that arises from these advantages.
Keywords
Introduction
Advances in laparoscopic technologies have greatly expanded the use of this technique in general surgery. The benefits of laparoscopic or minimally invasive surgery (better cosmesis, reduced postoperative pain, and faster recovery) are well known for many diseases, but reduced trauma to the abdominal wall is particularly evident in pancreatic surgery.[1,2] However, surgery of the pancreas is still challenging, and although the first reported case of laparoscopic approach in pancreatic disease was in 1994, it has been widely applied only in the past decade.[3-5] Open surgery is still performed because of the anatomy of pancreas, limitations of team skills, and some early concerns regarding oncologic outcomes.[6] Nevertheless, minimally invasive surgery has been increasingly adopted, particularly for benign or low-malignancy pancreatic tumours of the left pancreas. Several retrospective studies confirmed laparoscopic distal pancreatectomy (LDP) as a feasible and safe technique, even if no randomized controlled trials (RCTs) comparing open distal pancreatectomy (ODP) and LDP are available. Furthermore, it has been argued that costs for reduced hospital length of stay (LoS) are counterbalanced by the increased operative costs of LDP.[7]
The purpose of our study was to evaluate the safety of our standardized minimally invasive technique and assess if LDP is a cost-effective procedure compared to ODP.
Methods
Study design and population
The medical records of all patients treated for left-sided pancreatic lesions (with or without splenic preservation), between April 2013 and March 2015, at the Department of Oncologic Surgery at the Humanitas Gavazzeni Institute of Bergamo (Italy), were retrospectively analysed. Patients with both benign and malignant lesions were included in the study. Cases with insufficient data for analysis or that entailed simple tumour enucleation were excluded, as were those in which additional organ resections were performed during the same operation. All cases were discussed in a multidisciplinary gastrointestinal tumour board prior to surgery. Demographics and intraoperative and postoperative data were recorded in an ad hoc database.
The American Society of Anaesthesiologists (ASA) score was reported,[8] and body mass index (BMI) was calculated for each patient. Intraoperative blood loss, operative time, hospital LoS, postoperative morbidity, perioperative mortality (within 30 days from surgery), and 30-day readmission rates were also recorded. The level of pain reported was recorded three times per day on postoperative days 1 and 2, using the standard visual analogic scale (VAS). The presence of a postoperative pancreatic fistula was assessed according to the 2005 International Study Group on Pancreatic Fistula (ISGPF) criteria.[9] Analysis of costs included the expenses for the hospital stay, operative time and equipment (surgical staplers and energy devices), pharmaceutical treatment, nursing, and laboratory and pathology fees. No post-discharge care or home-nursing costs were included.
Surgical technique
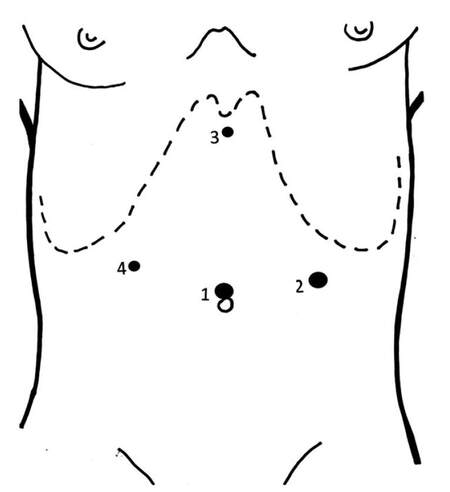
All pancreatic resections were performed by experienced surgeons using a standardized technique. The LDP patients were placed in the lithotomic position, with the operator placed between the patient’s legs. The operation was performed through four ports: umbilical, subxyphoid, and both subcostal positions in the mid-clavicular line so as to avoid trauma to the epigastric vessels [Figure 1]. The devices included a harmonic scalpel (Harmonic ACE®, Ethicon EndoSurgery, Cincinnati, OH, USA) used for dissection. Intraoperative ultrasound was used if needed to localize the tumour. In cases without splenic preservation, a vascular stapler was used to divide the splenic vein and two Hem-o-lok® (Teleflex Medical Europe Ltd., IDA Business and Technology Park, Athlone, Ireland) clips were applied on the splenic artery. Division of the pancreas was performed using a stapler. The specimen was placed in an Endopouch Retrieval Bag® (Ethicon EndoSurgery, Cincinnati, OH, USA) and removed through a slightly enlarged peri-umbilical incision or a Pfannenstiel incision for large specimens.
Figure 1. Position of trocar sites. (1) 10/12 mm umbilicus; (2) 10/12 mm left anterior axillary line between the costal margin and the iliac crest; (3) 5 mm subxiphoid area; (4) 5 mm lateral right rectus sheath under the right costal margin
For the open approach, patients were placed in the supine position and a left subcostal incision was used. The additional cost for the use of Harmonic Focus + Long Shears® (Ethicon EndoSurgery, Cincinnati, OH, USA) was calculated. The pancreatic stump was treated with a stapler, similarly to the laparoscopic approach.
A close suction drain was placed in all cases and removed when the presence of a pancreatic fistula was ruled out, according to the clinical and laboratory findings.
The enhanced recovery-after-surgery (ERAS) programme, including early oral intake, mobilization, and specific instructions for the management of drains and nasogastric tubes, was applied in all patients.[10]
Statistical analysis
The data are reported as mean ± standard deviation (SD). To compare continuous and dichotomized variables, we used the Mann-Whitney U-test (assuming that the data were not normally distributed) and the Fisher exact probability test (because most cell frequencies were ≤ 5), respectively. The relationship between parameters was evaluated using Pearson’s correlation coefficient calculation, and the relation line equations were also obtained. A P-value < 0.05 was considered statistically significant.
Results
Twelve patients (6 men and 6 women, median age 68, range 57 to 78 years) underwent LDP (group A), while 12 patients (5 men and 7 women, median age 71, range 59 to 79 years) underwent ODP (group B), for benign or malignant diseases.
Table 1 reports the main population characteristics and shows that the two groups were homogeneous (P = NS) with respect to age, male/female ratio, ASA score, and BMI. In addition, the pathological findings did not differ (P = NS) between groups. Pancreatic neuroendocrine tumour (NET) was the main diagnosis (5 in LDP group and 4 in ODP), followed by cystic tumours (2 serous and 1 mucinous in LDP vs. 2 serous and 2 mucinous in ODP). Other findings in the LDP group included two intraductal papillary mucinous neoplasms (IPMN), one hematoma, and a lymphoepithelial cyst, whereas one ductal adenocarcinoma, one IPMN, an epithelial cyst, and an inflamed pancreas were found in the ODP group. The intra- and postoperative results are displayed in Table 2. Both the size of the specimen (5.33 ± 3.2 vs. 5.58 ± 2.57 cm, P = 0.8033) and the number of the removed lymph nodes (10.5 ± 4.3 vs. 12.1 ± 3.1, P = 0.3071) were similar (P = NS). In three cases of LDP, the size of the lesion was more than 8 cm and required a Pfannenstiel incision for extraction of the surgical specimen. None of the patients in the LDP group were converted to an open approach.
Population's characteristics
| Parameters | Laparoscopic distal pancreatectomy | Open distal pancreatectomy | P-value |
|---|---|---|---|
| Number of patients | 12 | 12 | - |
| Age (years) | 68.08 ± 6.73 | 70.5 ± 6.9 | 0.2531 |
| Male/female ratio | 6:6 | 5:7 | 0.6801 |
| ASA | 2.08 ± 0.51 | 2.33 ± 0.49 | 0.2247 |
| BMI (range) | 26.92 ± 2.97 (24-35) | 27.83 ± 4.02 (22-37) | 0.7843 |
Intra- and postoperative results
| Results | Laparoscopic distal pancreatectomy | Open distal pancreatectomy | P-value |
|---|---|---|---|
| Length of surgery, min (range) | 197.5 ± 33.74 (160-285) | 122.5 ± 34.54 (90-215) | 0.00034 |
| Estimated blood loss, mL (range) | 100.83 ± 32.04 (60-180) | 180 ± 39.77 (120-250) | 0.0001 |
| Tumor size, cm (range) | 5.33 ± 3.2 (1.2-12.5) | 5.58 ± 2.57 (3-11) | 0.8033 |
| Number of removed lymph nodes (range) | 10.55 ± 4.3 (6-19) | 12.08 ± 3.12 (8-18) | 0.3071 |
| Post-operative VAS (on days I-II) | 4.08 ± 1.16 | 5.92 ± 1.24 | 0.0009 |
| Resumption of canalization, hours after surgery (range) | 48 ± 22.88 (24-96) | 92 ± 17.23 (72-120) | 0.001 |
| Resumption of solid oral feeding, days after surgery (range) | 2.42 ± 0.67 (2-4) | 3.4 ± 1.38 (2-6) | 0.1403 |
Laparoscopic pancreatectomy required a longer operative time (197.5 ± 33.7 vs. 122.5 ± 35.4 min, P = 0.00034). However, in this group of patients both postoperative pain intensity measured by a VAS scale (P = 0.0009) and the hospital stay (P = 0.0014) were significantly reduced, and the patients had an earlier bowel canalization (48 ± 23 vs. 92 ± 17 h, P = 0.001) [Table 2].
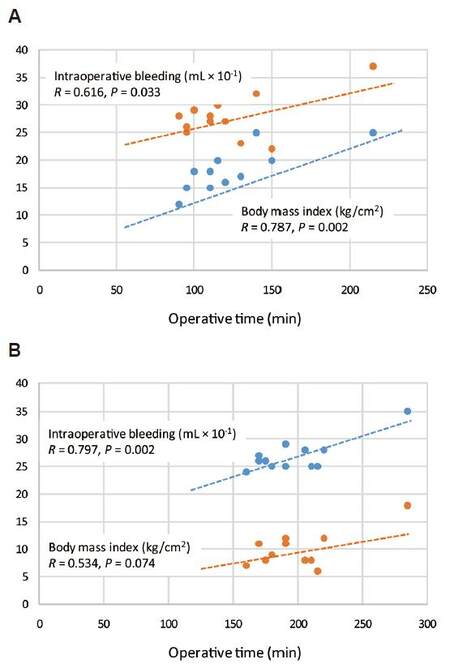
Table 3 summarizes correlations between operative time (OT) or hospital LoS and age, BMI, and intraoperative bleeding (IB), to evaluate whether there is any relationship between main variables. In both groups (LDP vs. ODP) the age did not affect operative time (R = 0.338, P = 0.226 vs. R = 0.9002, P = 0.996), which was related to the intraoperative bleeding (R = 0.797, P = 0.002 vs. 0.616, P = 0.003). A significant relationship between LoS and age (R = 0.578, P = 0.040) and between the operative time and BMI (R = 0.787, P = 0.002) was found only in group ODP [Figure 2A and B].
Correlations between OT or hospital LoS and age, BMI, and IB
| Parameters | ODP | LDP | ||||
|---|---|---|---|---|---|---|
| R | Regression line equation | P-value | R | Regression line equation | P-value | |
| OT/age | -0.0015 | Age = 68.6746 - 0.0029 OT | 0.9963 | 0.3378 | Age = 61.2656 + 0.0754 OT | 0.2259 |
| OT/BM I | 0.7873 | BMI = 13.2375 + 0.0692 OT | 0.0024 | 0.5337 | BMI = 20.2266 + 0.0620 OT | 0.0739 |
| OT/IB | 0.6159 | IB = 0.5848 OT - 14.6706 OT | 0.0330 | 0.7973 | IB = 67.5333 + 0.9181 OT | 0.0019 |
| LoS/age | 0.5780 | Age = 43.3988 + 2.4269 LoS | 0.0490 | 0.1556 | Age = 50.6381 + 1.5396 LoS | 0.6291 |
| LoS/OT | 0.4487 | OT = 17.1067 + 9.4382 LoS | 0.1434 | 0.5979 | OT = 110.7816 + 10.7280 LoS | 0.0399 |
| LoS/IB | 0.1482 | IB = 98.0898 + 5.8427 LoS | 0.6357 | 0.8284 | IB = 14.1113 - 3.2334 LoS | 0.0009 |
Figure 2. Relationship between operative time (min), body mass index (kg/cm2) and intraoperative bleeding (mL) in (A) laparoscopic distal pancreatectomy patients and (B) open distal pancreatectomy patients
The patients were started on a fluid diet on the 1st postoperative day and the diet was advanced to soft food as tolerated (LDP 2.4 ± 0.7 vs. ODP 3.4 ± 1.4 days). The hospital LoS was 8.1 ± 1.88 days (median 8, range 5 to 12) in LDP vs. 11.2 ± 1.6 (median 11, range 9 to 14) days in ODP, and the readmission rate was 8.3% in both groups. One patient in both groups developed a pancreatic fistula (Grade B), but no perioperative mortality occurred.
For a single LDP, a cost of €1,401 was calculated (energy devices, disposable trocars, and Endopouch), increased to €1,641 in cases of no spleen-preserving LDP (9 patients), which required a vascular cartridge and Haemoclips. For the open approach, an additional cost of €863 was required for the use of the Harmonic Focus, sutures, and more gauzes (€986 for non-spleen-preserving procedures). The global cost for LDP was €537 more than for an open surgery for each procedure. Calculating intra- and post-operative costs, we found an additional cost of €402 per patient for the minimally invasive technique.
The cost for a single day of hospital stay was on average €471 in our region (Lombardy). Thus, calculating the costs for the longer LoS in ODP vs. LDP (median 11.5 vs. 8.0), showed that there is a cost advantage favoring the minimally invasive approach vs. the open technique (€3,807.25 ± 885.92 vs. €5,259.5 ± 773.5). Table 4 summarizes LoS and total costs of patients who underwent LDP vs. ODP.
Hospital length of stay, median operative costs, and total costs according to the type of pancreatectomy
| Parameters | LDP | ODP | P-value |
|---|---|---|---|
| Hospital length of stay (days) | 8.08 ± 1.88 | 11.17 ± 1.64 | 0.0003 |
| Estimated median operative costs (Euros): | |||
| Spleen-preserving | 1,401 | 863 | |
| No spleen-preserving | 1,641 | 986 | |
| Estimated hospital stay costs (Euros): | |||
| Median (range) | 3,768 (2,355-5,652) | 5,416.5 (4,239-6,594) | |
| Mean | 3,807.25 ± 885.92 | 5,259.5 ± 773.5 | 0.0004 |
| Estimated median total costs (Euros) | 5,169 | 6,279.5 |
Discussion
The laparoscopic approach to pancreatic surgery has been utilized increasingly in the last decade, as a result of the evolution of minimally invasive technologies and the increasing numbers of pre-malignant and incidentally detected pancreatic lesions.[11] However, a population-based analysis on the Nationwide Inpatient Sample (NIS) found that, during the period 1998 to 2009, only 4.3% of distal pancreatectomies were performed with a minimally invasive approach.[12] Technical difficulties, due to the retroperitoneal location of the pancreas and the scarcity of high-volume skilled surgical teams, as well as the need to maintain oncologic standards, were major obstacles to the adoption of the laparoscopic approach.[5]
More recently, several studies and meta-analyses have shown that LDP is a safe procedure, with improved outcomes and reduced hospital stays.[13-16] Cao et al.[12] in their population-based retrospective cohort study reported a reduction of 1.22 days in LoS associated with minimally invasive surgery, with no differences in the perioperative mortality and total hospital costs. Furthermore, lower rates of infectious complications (30.1% vs. 39%) and bleeding complications (13.1% vs. 20.6%) were reported in LDP vs. ODP. Unfortunately, no randomized controlled trials (RCTs) comparing the two approaches are available, and all favourable results are reported in retrospective cohort-like or case-control studies.[17]
Our retrospective analysis was performed on a series of well-matched patients, with comparable demographics and similar histologic findings [Table 1]. In our experience, pancreatic NET and cystic tumours were the main result at definitive histology, and a distal minimally invasive pancreatic resection was the surgical approach of choice for such indolent malignancies. The treatment of these rare diseases requires expertise in both pancreatic surgery and advanced laparoscopy, but unfortunately, the number of retrospective reports is limited, and the complete information, including tumour size and margin status, are often missing.[18]
However, the progressive centralization of the surgical treatment of patients with pancreatic disease in specialized and high-volume centres will favour implementation of the procedure and data availability. A multicentre analysis, performed in 2010 by Kooby et al.,[6] reported similar oncologic results between LDP and ODP, with no differences in terms of overall survival and lymph node yield. Similarly, DiNorcia et al.[19] in 130 resections for PNET, reported no differences in morbidity, mortality, or overall survival (OS), between the laparoscopic and open approach.
An important issue concerning oncologic effectiveness of minimally invasive surgery is that of achieving microscopically negative margins (R0) and an adequate number of harvested lymph nodes. Several reports have addressed this topic, and different comparative studies found no significant differences of R0 rates between laparoscopic and open techniques (74-97% vs. 73-96%).[20] Abu Hilal et al.[21] reported a 76% of R0 and a median of 15 sample nodes, suggesting that their standardised technique was a reasonable and safe procedure in left-sided malignancies. Shin et al.[22] reported a rate of R0 resections of 82.9% in 152 left pancreatic lesions, with a median size of 3 cm, removed with minimally invasive access. Another recent series of distal pancreatectomies showed similar results in terms of R0/R1/R2 rates, and a median of 16 harvested lymph nodes in LDP vs. 14 in ODP.[13] Fernandez-Cruz et al.[23] performed 27 LDP, achieving an R0 resection in 90% of ductal adenocarcinomas, and removing a median of 6 lymph nodes in the LDP group vs. 8 in the ODP group. Interestingly, in our series, the number of removed lymph nodes was similar and adequate in both groups, despite benign and malignant diseases having been included (10.55 ± 4.3 vs. 12.08 ± 3.12, P = NS). Notably, data on pancreatic ductal adenocarcinoma suggest that a minimum of 12 lymph nodes should be excised to ensure adequate nodal assessment.[24] However, this point is still debated, and the oncologic effectiveness of the minimally invasive approach still worries some surgeons. In the USA, high-volume centres perform distal pancreatectomies with minimally invasive techniques, either laparoscopic or robotic, unless there are clear contraindications present. However, according to a recent survey, 31% of European surgeons still prefer ODP for oncologic purposes.[12,25]
In our series, ASA score and BMI were similar in the two groups and, in contrast to other studies, patients who were treated with LDP had similar tumour size as those treated with open approach (5.33 ± 3.2 vs. 5.58 ± 2.57 cm, P = NS). In previous studies, the laparoscopic approach was primarily used for small benign lesions or indolent malignancies. In a series of 360 distal pancreatic resections, 71 were totally laparoscopic but had a significantly smaller median tumor size (2.5 cm in LDP vs. 3.6 cm in the ODP group).[26] Similarly, another systematic review reported a mean tumor size of 3.5 cm in LDP vs. 3.9 cm in ODP.[27] In our experience, the size of the specimen was not a contraindication or a major obstacle to laparoscopic approach, but had an impact on the duration of surgical intervention. It is noteworthy that there is a recent trend toward an increased size of the excised lesions (4.0 ± 2.8 cm vs. 3.3 ± 1.5 cm) noted in the literature.[20]
Consistent with previous studies,[14,15] our operative time for LDP was longer than for ODP (median 195.5 vs. 112.5 min). Shin et al.[22] reported a median operative time of 195 min for LDP, whereas Braga et al.[28] reported a median duration of surgery of 239 min for LDP, significantly higher than that for ODP (213 min), but their series included a high rate (30%) of adenocarcinomas. Another group reported a longer operative time for LDP (376 min vs. 274 min).[29] In our series, the higher operative time was related to one operation (285 min) in an obese patient with diffuse adhesions, and three cases in which the size of the specimens necessitated a Pfannenstiel incision, also lengthening the duration of surgery. Interestingly, we found that in both groups age did not affect operative time, which was related to intraoperative bleeding, whereas a significant relationship between the operative time and BMI only occurred in the ODP group.
Undoubtedly, standardization of the technique and expertise of the surgical team is crucial. Another systematic review found no difference in operative time on 488 patients treated laparoscopically and 573 cases with open approach (mean 220.4 vs. 208.6 min).[27]
In agreement with previous studies and meta-analyses, we encountered lower intraoperative blood loss in the minimally invasive group. A wide population-based analysis reported a lower rate of bleeding complications in LDP (13.1% vs. 20.6%) and also a reduction of transfusion rate (11.3% vs. 18%).[12] However, the reported blood loss varies widely between studies, and may be related to the surgical technique or to the accuracy of the quantification of the bleeding. Jusoh et al.[27] reported a mean operative blood loss of 237.4 mL in LDP versus 562.4 mL in ODP, whereas Limongelli et al.[30] found a blood loss of 160 ± 185 mL vs. 365 ± 215 mL, respectively. Interestingly, Rutz et al.[7] reported an estimated blood loss of 113 ± 155 mL in LDP vs. 210 ± 274 mL in ODP, further differentiating blood loss between a totally laparoscopic approach (LDP, 76 ± 71 mL) and laparoscopic hand-assisted distal pancreatectomy (LHDP, 197 ± 244 mL). Very recently, a meta-analysis of short-term outcomes between LDP and robotic-assisted distal pancreatectomy (RADP) found a lower blood loss and a higher rate of spleen-preserving procedures in RADP.[31] Thus, the technological improvements and the magnified view during laparoscopy are crucial for control of bleeding. Nevertheless, lower rates of bleeding where found in a surgical series that excluded malignancies, suggesting a major role for the size and histology of the tumor.[32]
Concerning morbidity, a large population-based analysis reported a 25% reduction of overall perioperative complications, particularly related to a lower rate of postoperative infections (30.1% vs. 39%) and bleeding complications (13.1% vs. 20.6%).[12] Similarly, Venkat et al.[14] reported a reduction in overall morbidity after the minimally invasive approach (33.9% vs. 44.2%), including a lowering of the percentage of surgical site infections (2.9% vs. 8.1%).However, most of the reports found no differences in complication rates between the two approaches.[13] Magge et al.[33] reported equal rates and severity of complications (39% vs. 50%) in 62 patients undergoing distal pancreatectomy for early-stage ductal adenocarcinoma, and found that conversion to an open procedure was associated with poor outcome. Similarly, Jayaraman et al.[34] compared 343 LDP vs. 236 ODP and found that patients who required conversion had more complications and pancreatic leaks. These findings confirm the need for accurate preoperative patient selection, to identify patients at high risk for conversion and to choose the best approach for each patient and disease presentation.
Post-operative pancreatic fistulas (POPF) remain the most feared complication, but the incidence is variable among different surgeons, partly because of different definitions of POPF. We strictly applied the International Study Group for Pancreatic Fistulae (ISGPF) definition of POPF and, considering only grade B and C fistulae, we found no differences between the two groups, with one case of POPF in both (8.3%).[9] A large multicenter study, using the same ISGPF criteria, found no difference in pancreatic leaks between the laparoscopic and the open approach.[35] Similarly, a meta-analysis of 18 studies reported a similar incidence of grade B-C fistulae after either the laparoscopic or the open approach.[14] Velanovich et al.[36] reported a rate of POPF of 13% in both groups, whereas Kooby et al.[37] reported 26% POPF in LDP and 32% in ODP. Another series showed 14% POPF in LDP (n = 70) vs. 13% after open surgery (n = 45), similar to the report of Corcione et al.[38] (10.4% in LDP). In contrast, Fox et al.[39] reported a higher incidence of POPF in LDP (28.57%) compared to ODP (13.16%), but LDP led to only grade A fistulae, while all the grade B-C fistulae occurred in the ODP group. The occurrence of POPF varies widely between surgeons, and this may be attributable to the criteria adopted for definition rather than to the surgical technique. A meta-analysis of the most popular techniques (sutures, stapled closure, combination of both, with or without fibrin glue) did not identify one as being the most safe.[40] A multicenter RCT performed in 21 European hospitals found that hand-sewn sutures and closure with stapler were equally effective after distal pancreatectomy, but the identification and suture of a transected pancreatic duct is the only technique able to reduce the incidence of POPF.[41,42] Our standardized technique and the use of a stapled closure of the pancreatic remnant, despite the low number of patients, has proven to be safe, without significant morbidity or mortality, and with similar re-admission rates between groups.
In our experience the LDP group showed reduced pain intensity measured on the standard VAS scale (median 4 vs. 6) during the first 2 postoperative days, allowing reduced use of analgesics and earlier mobilization. Similarly, resumption of bowel canalization (median 48 vs. 96 h) and solid oral feeding (median 2 vs. 3 days) were shortened with LDP, compared with ODP.
An ERAS protocol was applied in all patients, as previously reported.[10] These programs, introduced for colorectal surgery, have been progressively adopted by other surgical specialities, leading to a reduction of postoperative morbidity and a shortening of LoS.[43] Pancreatic surgery is still a high risk procedure, but several non-randomized trials have demonstrated that ERAS in pancreatic resections is safe and feasible.[44] In our study, the use of minimally invasive surgery together with recommendations of the ERAS programme have shown complementary roles, speeding recovery and shortening LoS.
Consistent with previous studies, the hospital LoS was significantly reduced in patients treated with minimally invasive approach (median 8 vs. 11.5 days in LDP and ODP groups, respectively). Venkat et al.[14] found a 4 days reduction in LoS with LDP, whereas Cao et al.[12] in a large population-based analysis, reported a mean LoS of 8.62 days in the laparoscopic group vs. 10.76 days in the open one. Pericleous et al.[15] in their meta-analysis of case-matched studies, reported a reduced LoS of 2.7 days, similar to other groups, who reported a reduction of LoS of 2.7 to 5 days for LDP, compared with ODP. Very recently, a Cochrane review found that mean LoS was shorter by 2.43 days in the minimally invasive group compared with the open surgery group.[17] Hospital stay is considered an important evaluation index in laparoscopic surgery. Thus, our finding is interesting and probably related to the implementation of ERAS protocol, with earlier weaning from i.v. analgesia and earlier canalization. Interestingly, a significant relationship between LoS and age was found only in the ODP group.
Cost effectiveness of a procedure has become important, given that resources are limited and cost control is necessary, particularly in the Italian health system. Unfortunately, our analysis is not generalizable to different countries, because of variations in the different health systems’ reimbursements and practices.[12] A simplistic trade-off between operative costs and LoS may lead to a rough estimate, resulting in higher cost for the minimally invasive approach.[45] Furthermore, technologic advances and availability of new stapler and vessel-sealing devices, have improved the minimally invasive approaches, but simultaneously increased the costs of the procedure. We found an additional cost of €537 for each minimally invasive distal pancreatectomy, compared with a traditional open operation (see Results). However, in their meta-analysis, Nigri et al.[46] argued that devices are often equally used in LDP and ODP, but other practices or habits may influence results.A Korean single-centre study found significantly higher total costs for LDP, but LoS in this series was higher than any other published study (11.5 ± 4.1 days for LDP and 13.5 ± 4.9 days for ODP), reflecting the importance of different practices.[47] All subsequent studies found that although the operative costs were higher for minimally invasive procedures, decreased LoS after laparoscopic resection balanced, at least, overall costs.[30,48] Rutz et al.[7] found a mean operative cost of $4,900 for ODP and $5,756 for LDP, but calculated a mean total cost of care of $13,900 for the open procedure vs. $10,480 for the laparoscopic one. In this study, we accurately evaluated the overall expenses of the procedures, calculating device, equipment and all disposable costs as electronically cataloged. Similar to other studies, we calculated the costs for a longer LoS in ODP vs. LDP (median 11.5 vs. 8 days), and we found an advantage of costs for the hospital stay favoring the minimally invasive approach vs. the open technique (mean cost, €5,169 vs. €6,279.5).
Undoubtedly, reduction of hospital stay impacts expenses, lowering the overall cost of postoperative management. Furthermore, the minimally invasive approach contributes to reduction of postoperative pain and earlier ambulation, favouring an earlier discharge of patients. Consistent with our findings, Fox et al.[39] found a shorter LoS and a reduction of total hospital costs for LDP (n = 42), compared with ODP (n = 76), showing that LoS was directly proportional to total costs. Interestingly, Braga et al.[28] suggested that the cost-benefit analysis should consider not only the hospital charges, but also the cosmesis and quality of life of the patients to fully evaluate the minimally invasive approach. Notably, in our study the postoperative complications and readmission rate were similar. Ahmad et al.[49] found that postoperative complications and higher transfusion requirements, or the presence of chronic pancreatitis, had a significant impact on 30- and 90-day readmission rates.
Our study has several limitations. The main one is that it utilized retrospective data, which may introduce selection bias and allow missing information. Demographics, histology, and tumour size were similar in both groups, despite the absence of randomization. However, the number of patients in our series was low, but all available studies are similar cohort-like or case-control studies from single centres, with few patients. Unfortunately, no long-term data are available in our series, but a lack of long-term results and follow-up is common, as a result of the rarity of this type of disease and heterogeneity of the studies. Particularly, long-term data on recurrence of pancreatic carcinomas are scarce, and larger comparative studies are needed.[20]
Rehman et al.[28] found no significant differences in 3-year OS between laparoscopic (n = 8) or open (n = 14) distal pancreatectomy for pancreatic adenocarcinoma (82% vs. 74%). Similarly, Hu et al.[50] compared 11 LDP and 23 ODP and found a mean OS 42.0 ± 8.6 months vs. 54.0 ± 5.8 months. The Central Pancreas Consortium reported the same median OS (16 months) after both procedures, in matched cohorts, suggesting that oncologic outcomes are similar and independent of the surgical approach.[6]
In conclusion, our experience confirms that the minimally invasive surgical treatment of tumours of the distal pancreas is safe and feasible. Laparoscopic pancreatectomy offers several advantages compared with open surgery, including a significant reduction of estimated blood loss, reduced postoperative pain intensity, and earlier bowel canalization. However, implementation of minimally invasive pancreatectomy requires specific skills and adequate training, both in advanced laparoscopic surgery and in pancreatic surgery. Additional research and adequate RCTs are needed before the technique can be considered the “gold-standard” for distal pancreatectomies, to assess oncologic results and long-term outcomes. Finally, the costs of laparoscopic surgery should be carefully re-evaluated before concluding that they are greater than those of open surgery.
Authors’ contributions
Designed the study: S.M.M. Basso, P. Ubiali
Acquired the data: F. Maffeis, A. Patanè
Analyzed and interpreted the results: S.M.M. Basso, F. Maffeis, F. Lumachi
Drafted the manuscript: S.M.M. Basso
Revised the manuscript: S.M.M. Basso, F. Lumachi, M. Ciocca Vasino, P. Ubiali
Approved the final version: S.M.M. Basso, F. Maffeis, F. Lumachi, A. Patanè, M. Ciocca Vasino, P. Ubiali
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
Patient consent
Informed consent was obtained from all patients.
Ethics approval
The present study is a retrospective review of anonymized clinical records, and ethical permission was obtained.
REFERENCES
1. Tiwari MM, Reynoso JF, High R, Tsang AW, Oleynikov D. Safety, efficacy, and cost-effectiveness of common laparoscopic procedures. Surg Endosc 2011;25:1127-35.
2. Brokelman WJ, Lensvelt M, Borel Rinkes IH, Klinkenbijl JH, Reijnen MM. Peritoneal changes due to laparoscopic surgery. Surg Endosc 2011;25:1-9.
3. Soper NJ, Brunt LM, Dunnegan DL, Meininger TA. Laparoscopic distal pancreatectomy in the porcine model. Surg Endosc 1994;8:57-60.
5. Takaori K, Tanigawa N. Laparoscopic pancreatic resection: the past, present, and future. Surg Today 2007;37:535-45.
6. Kooby DA, Hawkins WG, Schmidt CM, Weber SM, Bentrem DJ, Gillespie TW, Sellers JB, Merchant NB, Scoggins CR, Martin RC 3rd, Kim HJ, Ahmad S, Cho CS, Parikh AA, Chu CK, Hamilton NA, Doyle CJ, Pinchot S, Hayman A, McClaine R, Nakeeb A, Staley CA, McMasters KM, Lillemoe KD. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg 2010;210:779-85.
7. Rutz DR, Squires MH, Maithel SK, Sarmiento JM, Etra JW, Perez SD, Knechtle W, Cardona K, Russell MC, Staley CA 3rd, Sweeney JF, Kooby DA. Cost comparison analysis of open versus laparoscopic distal pancreatectomy. HPB 2014;16:907-14.
8. ASA Physical Status Classification System. Last approved by the ASA House of Delegates on October 15, 2014. Available at: https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. [Last accessed on May 31, 2017].
9. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13.
10. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630-41.
11. Taylor C, O'Rourke N, Nathanson L, Martin I, Hopkins G, Layani L, Ghusn M, Fielding G. Laparoscopic distal pancreatectomy: the Brisbane experience of forty-six cases. HPB (Oxford) 2008;10:38-42.
12. Tran Cao HS, Lopez N, Chang DC, Lowy AM, Bouvet M, Baumgartner JM, Talamini MA, Sicklick JK. Improved perioperative outcomes with minimally invasive distal pancreatectomy: results from a population-based analysis. JAMA Surg 2014;149:237-43.
13. Joliat GR, Demartines N, Halkic N, Petermann D, Schafer M. Short-term outcomes after distal pancreatectomy: laparotomy vs. laparoscopy -- a single-center series. Ann Med Surg 2017;13:1-5.
14. Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59.
15. Pericleous S, Middleton N, McKay SC, Bowers KA, Hutchins RR. Systematic review and meta-analysis of case-matched studies comparing open and laparoscopic distal pancreatectomy: is it a safe procedure? Pancreas 2012;41:993-1000.
16. Melotti G, Butturini G, Piccoli M, Casetti L, Bassi C, Mullineris B, Lazzaretti MG, Pederzoli P. Laparoscopic distal pancreatectomy: results on a consecutive series of 58 patients. Ann Surg 2007;246:77-82.
17. Riviere D, Gurusamy KS, Kooby DA, Vollmer CM, Besselink MG, Davidson BR, van Laarhoven CJ. Laparoscopic versus open distal pancreatectomy for pancreatic cancer. Cochrane Database Syst Rev 2016;4:CD011391.
18. Kooby DA. Tips and tricks of laparoscopic distal pancreatectomy for ductal adenocarcinoma. J Hepatobiliary Pancreat Sci 2016;23:E10-3.
19. DiNorcia J, Lee MK, Reavey PL, Genkinger JM, Lee JA, Schrope BA, Chabot JA, Allendorf JD. One hundred thirty resections for pancreatic neuroendocrine tumor: evaluating the impact of minimally invasive and parenchyma-sparing techniques. J Gastrointest Surg 2010;14:1536-46.
20. Postlwait LM, Kooby DA. Laparoscopic distal pancreatectomy for adenocarcinoma: safe and reasonable? J Gastrointest Oncol 2015;6:406-17.
21. Abu Hilal M, Richardson JR, de Rooij T, Dimovska E, Al-Saati H, Besselink MG. Laparoscopic radical 'no-touch' left pancreatosplenectomy for pancreatic ductal adenocarcinoma: technique and results. Surg Endosc 2016;30:3830-8.
22. Shin SH, Kim SC, Song KB, Hwang DW, Lee JH, Park KM, Lee YJ. Appraisal of laparoscopic distal pancreatectomy for left-sided pancreatic cancer: a large volume cohort study of 152 consecutive patients. PLoS One 2016;11:e0163266.
23. Fernández-Cruz L, Poves I, Pelegrina A, Burdío F, Sánchez-Cabus S, Grande L. Laparoscopic distal pancreatectomy for pancreatic tumors: does size matter? Dig Surg 2016;33:290-8.
24. Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, Choti MA, Pawlik TM. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165-74.
25. de Rooij T, Besselink MG, Shamali A, Butturini G, Busch OR, Edwin B, Troisi R, Fernández-Cruz L, Dagher I, Bassi C, Abu Hilal M; DIPLOMA trial group. Pan-European survey on the implementation of minimally invasive pancreatic surgery with emphasis on cancer. HPB 2016;18:170-6.
26. DiNorcia J, Schrope BA, Lee MK, Reavey PL, Rosen SJ, Lee JA, Chabot JA, Allendorf JD. Laparoscopic distal pancreatectomy offers shorter hospital stays with fewer complications. J Gastrointest Surg 2010;14:1804-12.
27. Jusoh AC, Ammori BJ. Laparoscopic versus open distal pancreatectomy: a systematic review of comparative studies. Surg Endosc 2012;26:904-13.
28. Braga M, Pecorelli N, Ferrari D, Balzano G, Zuliani W, Castoldi R. Results of 100 consecutive laparoscopic distal pancreatectomies: postoperative outcome, cost-benefit analysis, and quality of life assessment. Surg Endosc 2015;29:1871-8.
29. Rehman S, John SKP, Lochan R, Jaques BC, Manas DM, Charnley RM, French JJ, White SA. Oncological feasibility of laparoscopic distal pancreatectomy for adenocarcinoma: a single-institution comparative study. World J Surg 2014;38:476-83.
30. Limongelli P, Belli A, Russo G, Cioffi L, D'Agostino A, Fantini C, Belli G. Laparoscopic and open surgical treatment of left-sided pancreatic lesions: clinical outcomes and cost-effectiveness analysis. Surg Endosc 2012;26:1830-6.
31. Zhou JY, Mou C, Mou YP, Xu XW, Zhang MZ, Zhou YC, Lu C, Chen RG. Robotic versus laparoscopic distal pancreatectomy: a meta-analysis of short-term outcomes. PLoS One 2016;11:e0151189.
32. Teh SH, Tseng D, Sheppard BC. Laparoscopic and open distal pancreatic resection for benign pancreatic disease. J Gastrointes Surg 2007;11:1120-5.
33. Magge D, Gooding W, Choudry H, Steve J, Steel J, Zureikat A, Krasinskas A, Daouadi M, Lee KK, Hughes SJ, Zeh HJ 3rd, Moser AJ. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg 2013;148:525-31.
34. Jayaraman S, Gonen M, Brennan MF, D'Angelica MI, DeMatteo RP, Fong Y, Jarnagin WR, Allen PJ. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 2010;211:503-9.
35. Cho CS, Kooby DA, Schmidt CM, Nakeeb A, Bentrem DJ, Merchant NB, Parikh AA, Martin RC 2nd, Scoggins CR, Ahmad SA, Kim HJ, Hamilton N, Hawkins WG, Weber SM. Laparoscopic versus open left pancreatectomy: can preoperative factors indicate the safer technique? Ann Surg 2011;253:975-80.
36. Velanovich V. Case-control comparison of laparoscopic versus open distal pancreatectomy. J Gastrointest Surg 2006;10:95-8.
37. Kooby DA, Gillespie T, Bentrem D, Nakeeb A, Schmidt MC, Merchant NB, Parikh AA, Martin RC 2nd, Scoggins CR, Ahmad S, Kim HJ, Park J, Johnston F, Strouch MJ, Menze A, Rymer J, McClaine R, Strasberg SM, Talamonti MS, Staley CA, McMasters KM, Lowy AM, Byrd-Sellers J, Wood WC, Hawkins WG. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg 2008;248:238-46.
38. Corcione F, Marzano E, Cuccurullo D, Caracino V, Pirozzi F, Settembre A. Distal pancreas surgery: outcome for 19 cases managed with a laparoscopic approach. Surg Endosc 2006;20:1729-32.
39. Fox AM, Pitzul K, Bhojani F, Kaplan M, Moulton CA, Wei AC, McGilvray I, Cleary S, Okrainec A. Comparison of outcomes and costs between laparoscopic distal pancreatectomy and open resection at a single center. Surg Endosc 2012;26:1220-30.
40. Knaebel HP, Diener MK, Wente MN, Büchler MW, Seiler CM. Systematic review and meta-analysis of technique for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg 2005;92:539-46.
41. Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G, Tomazic A, Bruns CJ, Busch OR, Farkas S, Belyaev O, Neoptolemos JP, Halloran C, Keck T, Niedergethmann M, Gellert K, Witzigmann H, Kollmar O, Langer P, Steger U, Neudecker J, Berrevoet F, Ganzera S, Heiss MM, Luntz SP, Bruckner T, Kieser M, Büchler MW. Efficacy of stapler versus hand-sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet 2011;377:1514-22.
42. Bilimoria MM, Cormier JN, Mun Y, Lee JE, Evans DB, Pisters PW. Pancreatic leak after left pancreatectomy is reduced following main pancreatic duct ligation. Br J Surg 2003;90:190-6.
43. Barton JG. Enhanced recovery pathways in pancreatic surgery. Surg Clin North Am 2016;96:1301-12.
45. Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, Wolfe BM. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg 2001;234:279-89; discussion 289-91.
46. Nigri GR, Rosman AS, Petrucciani N, Fancellu A, Pisano M, Zorcolo L, Ramacciato G, Melis M. Metaanalysis of trials comparing minimally invasive and open distal pancreatectomies. Surg Endosc 2011;25:1642-51.
47. Eom BW, Jang JY, Lee SE, Han HS, Yoon YS, Kim SW. Clinical outcomes compared between laparoscopic and open distal pancreatectomy. Surg Endosc 2008;22:1334-8.
48. Abu Hilal M, Hamdan M, Di Fabio F, Pearce NW, Johnson CD. Laparoscopic versus open distal pancreatectomy: a clinical and cost-effectiveness study. Surg Endosc 2012;26:1670-4.
49. Ahmad SA, Edwards MJ, Sutton JM, Grewal SS, Hanseman DJ, Maithel SK, Patel SH, Bentram DJ, Weber SM, Cho CS, Winslow ER, Scoggins CR, Martin RC, Kim HJ, Baker JJ, Merchant NB, Parikh AA, Kooby DA. Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg 2012;256:529-37.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Basso SMM, Maffeis F, Lumachi F, Patanè A, Vasino MC, Ubiali P. Mini-invasive distal pancreatectomy: a feasible and cost-effective technique. Mini-invasive Surg 2017;1:133-42. http://dx.doi.org/10.20517/2574-1225.2017.06
AMA Style
Basso SMM, Maffeis F, Lumachi F, Patanè A, Vasino MC, Ubiali P. Mini-invasive distal pancreatectomy: a feasible and cost-effective technique. Mini-invasive Surgery. 2017; 1: 133-42. http://dx.doi.org/10.20517/2574-1225.2017.06
Chicago/Turabian Style
Basso, Stefano Maria Massimiliano, Federica Maffeis, Franco Lumachi, Alessandro Patanè, Michele Ciocca Vasino, Paolo Ubiali. 2017. "Mini-invasive distal pancreatectomy: a feasible and cost-effective technique" Mini-invasive Surgery. 1: 133-42. http://dx.doi.org/10.20517/2574-1225.2017.06
ACS Style
Basso, SMM.; Maffeis F.; Lumachi F.; Patanè A.; Vasino MC.; Ubiali P. Mini-invasive distal pancreatectomy: a feasible and cost-effective technique. Mini-invasive. Surg. 2017, 1, 133-42. http://dx.doi.org/10.20517/2574-1225.2017.06
About This Article
Copyright
Data & Comments
Data

 Cite This Article 2 clicks
Cite This Article 2 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.