Current state of transanal minimally invasive surgery in the management of rectal cancer
Abstract
Rectal cancer surgery has undergone a rapid change over the last few decades. We have come a long way from abdominoperineal resection to minimally invasive sphincter preserving techniques. Colorectal cancer screening programs made it possible to diagnose patients at earlier stages and this has led to question the necessity of radical surgery and the possibility of organ preservation. The platform most recently added to the surgical armamentarium is transanal minimally invasive surgery (TAMIS). It utilizes conventional laparoscopic tools to perform endoluminal surgery in rectum. Along with the conceptual changes in rectal cancer management, TAMIS is more frequently used for local excision of malignant rectal tumors. This review highlights the recent advances and current state of the role of TAMIS in the management of rectal cancer at various stages.
Keywords
Introduction
The ultimate aim of rectal cancer treatment is to provide safe oncological cure while maintaining enteral continuity and preserving sphincter function. In many cases, it is challenging to achieve excellent results in all three components[1].
The multimodal treatment of rectal cancer is following a similar path to breast cancer: less invasive surgical techniques are being utilized to preserve anatomical and functional integrity without compromising oncological outcomes. It has undergone a seismic change from abdominoperineal resection to low anterior resection, local excision and finally watch-and-wait approach, following neoadjuvant treatment in select patients. This change was manifest in tandem to the technical advancements like the introduction of circular staplers, surgical refinements - popularization of strict adherence to anatomical planes by Heald, and of course recognition of the importance of neoadjuvant chemotherapy and radiotherapy[2].
Not only has the nomenclature around the operation changed, but so too has the platform to access the rectum. The introduction of laparoscopy has revolutionised colorectal surgical practice and continues to evolve. Robotic surgery platforms have also garnered some popularity, in particular, for access to the lower third of the rectum. Since 2010, the introduction of minimally invasive approaches has been applied to the rectum via a transanal approach[3], and has been utilised in a broad spectrum of clinical scenarios; from transanal polyp excision to anastomotic leak repair, local excision of rectal cancer, transanal total mesorectal excision (taTME) and pelvic exenteration[4-9].
The advent of widespread colorectal cancer screening has made it possible to diagnose rectal polyps and early stage rectal cancers more frequently. This increasing trend along with increasing response rates to more effective neoadjuvant treatment for locally advanced rectal cancers and patient demands for organ-sparing options has led leaders in the field to push the boundaries of surgical approaches to the rectum and to reappraise the paradigm of formal proctectomy. Moreover, patients who require local palliation in the setting of stage IV disease and the aging population with medical comorbidities who would be otherwise unfit for any abdominal approach, constitute another group of patients for whom local interventions per anus may prove to be more beneficial overall.
The aim of this study is to review the current state of the role of transanal minimally invasive surgery (TAMIS) in the local management of rectal cancer and highlight the recent advances, with an emphasis on functional results and complications.
Local excision
The term “local excision” refers to removal of the tumor with negative surgical margins without removing the organ it originates from - the rectum. It involves full thickness resection of the rectal wall but not necessarily the draining lymphatics. Enthusiasm about local excision for early stage rectal cancer has grown after Morson et al.[10] published their results in 1977. This has led to development of techniques other than transanal excision (TAE), which is limited by poor exposure and limited to lesions in the distal rectum. Currently, the two most popular options for local excision are transanal endoscopic microsurgery (TEM) and TAMIS.
TAE utilizes conventional instruments under direct vision. It cannot reach mid- or upper rectal lesions. Moreover, confinement of the operative field risks the achievement of negative surgical margins. Margin positivity exceeds 10% even in experienced hands[11,12]. In a recent study, TAE is not considered as a feasible technique for tumors located higher than the first rectal valve, > 3 cm in size and deeper than T1[13].
TEM, first described in 1984 by Buess et al.[14] utilized a rigid platform to access intraluminal lesions in the rectum. It has several advantages over TAE. It maintains a stable pneumorectum and makes it possible to reach the mid and upper rectum. Improved visualization results in better assessment of resection margins. When compared to conventional TAE, TEM provides a superior quality resection, with higher rates of negative microscopic margins, reduced rates of specimen fragmentation and lesion recurrence, but with equivalent post-operative complications[15].
However, several factors have limited the widespread uptake within the armamentarium of colorectal surgeons throughout the world. These include a steep learning curve, significant cost of the operating system and concerns about postoperative function[16].
The need for an oncologically safe and also cost effective procedure led to evolution of TAMIS. TAMIS utilizes conventional laparoscopic devices and a single incision port rather than a specialized platform. Therefore, it lowers the cost of the procedure, while giving the surgeon an opportunity to operate with familiar instruments. It also allows for a 360° exposure of the rectal lumen, which is another superiority over TEM. While TEM requires repositioning of the patient or the platform, TAMIS allows operating in multiple quadrants using the same configuration. First described in 2010, TAMIS was found to be a feasible alternative to TEM, providing its benefits at a fraction of the cost without specialized instrumentation[1,3,17-19].
An individualized approach for patients
Patients with rectal cancer being considered for local excision, should undergo routine staging workup like any other rectal cancer patient, including dedicated magnetic resonance imaging of rectum for local staging, computed tomography of chest, abdomen and pelvis to screen for distant metastases and baseline carcinoembryonic antigen level to guide future follow-up and treatment.
Neoadjuvant treatment followed by TME is still considered the gold standard treatment for locally invasive rectal cancer in terms of oncological outcomes. However, it also has significant effects on patients’ quality of life. The Dutch Colorectal Cancer Group reported 14% fecal incontinence, 52% bowel dysfunction and 57% urinary incontinence at 5-year follow-up[20]. These relatively high morbidity rates have strengthened the search for alternative treatment options providing a balance of favourable functional outcomes without compromising oncological results.
The uptake of colorectal cancer screening has enabled more patients to be diagnosed at an early stage[2]. We have also more knowledge about tumor biology and risk factors for aggressive behaviour of the disease. All of these together, bring up the potential for less radical organ preserving surgery in an effort to improve patients’ quality of life. One concern for local excision is excessive tissue removal leading to a narrowed lumen and rectal stenosis. However, we know from TEM literature that stenosis following TEM excision is rare unless the lesion is circumferential. Recently, McLemore et al.[21] reported a single case of rectal stenosis in a cohort of 32 patients who underwent TAMIS for both benign and malignant rectal tumors. The patient who developed stenosis had a large circumferential adenoma and was subsequently managed successfully with endoscopic dilation.
T1N0
Early rectal cancer is defined as cancer confined to submucosa[22]. Kikuchi et al.[23] further classified early rectal cancer according to depth of invasion of the tumor by dividing submucosal layer into thirds. While the risk of lymph node metastasis is 3% for lesions invading the superficial 1/3 of submucosa (SM1), it rises up to 8% for middle (SM2) and to 23% for deeply invading lesions (SM3)[24-26].
The 2013 American Society of Colon and Rectal Surgeons practice parameters for the management of rectal cancer state that local excision is an appropriate treatment modality for carefully selected T1 rectal cancers without high-risk features[27].
Favourable T1 lesions have a less than 10% risk of lymph node metastasis and local excision can be potentially curable for these patients[2][Figures 1-3]. The Swedish Rectal Cancer Registry analysis demonstrated that the rate of lymph node metastasis is 6% in the absence of adverse features (lymphovascular invasion or poor differentiation).
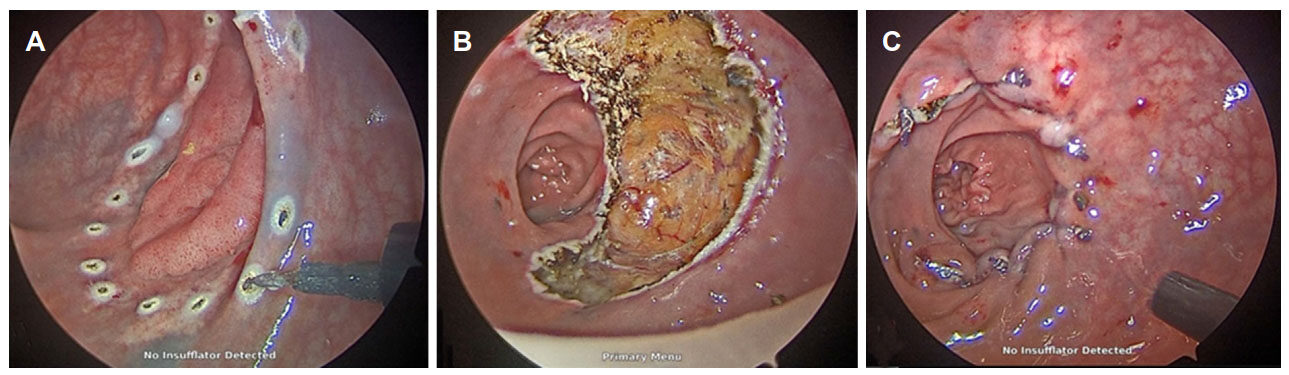
Figure 1. A T1 SM1 tumor excised by transanal minimally invasive surgery. A: Marking of resection margins with electrocautery; B: completed full thickness excision; C: closure of rectal wall defect
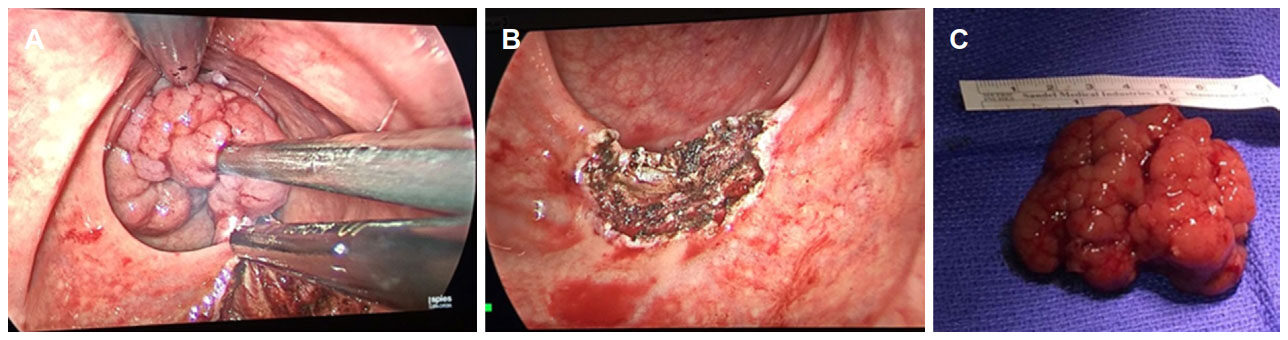
Figure 2. A near-obstructing T1 malignant polyp with a thin stalk excised by transanal minimally invasive surgery. A: Large tumor in mid rectum; B: rectal wall defect not closed after excision; C: the specimen after excision
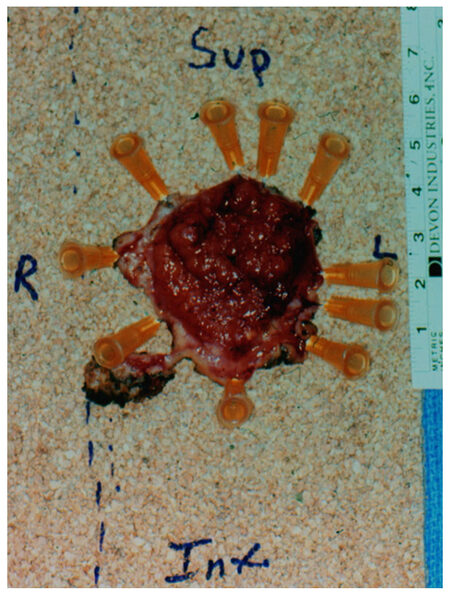
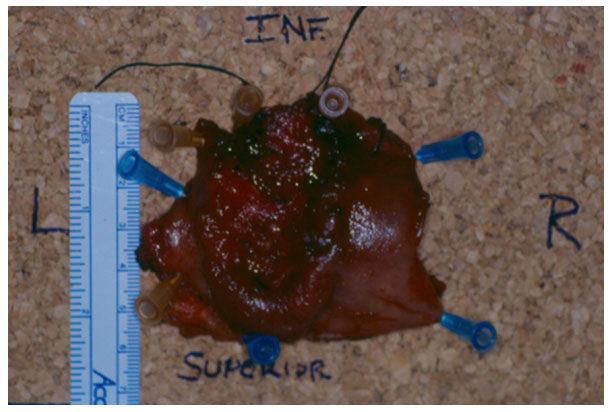
Figure 3. A T1 tumor excised with clear margins fixed on the board after transanal minimally invasive surgery excision
A recent analysis of Surveillance, Epidemiology, and End Results database, showed that local excision of T1 rectal cancer does not affect cancer-specific survival when compared to radical surgery. However, less radical approach comes at a cost of need for more frequent and careful follow-ups[28].
Due to the fact that TAMIS is a novel technique, oncological outcome data after TAMIS for rectal cancer is limited thus far simply by length of follow-up. In a comparative study by Lee et al.[29], margin positivity was 7% and lesion fragmentation was 4% for TAMIS, which was not significantly different from TEM. Local recurrence was 6% after high quality excision compared with 13% after poor quality excision. Another study comprising 110 rectal cancer patients, a positive margin was seen in 8% and tumor fragmentation in 5% of patients. For patients who did not undergo immediate salvage radical surgery, local recurrence rate was 6%, and distant metastasis rate was 2% after a median follow-up 14.4 months[30]. Martin-Perez et al.[31] performed a systemic review of 16 high quality case series, they reported an overall margin positivity of 4.4% and tumor fragmentation was 4.1%.
On the other hand, another meta-analysis of 4510 patients highlighted the risk factors for lymph node metastasis for T1 lesions as submucosal invasion > 1 mm, lymphovascular invasion, poor differentiation and tumor budding[32]. If any of these risk factors are present on final pathology, total mesorectal excision is recommended in medically fit patients due to the high risk of lymph node metastasis. Similarly, for rectal neuroendocrine tumors > 20 mm or with adverse features, radical surgery is warranted in suitable patients[1,15]. Data from TEM literature suggest a reduction in mesorectal excision quality in patients who undergo salvage radical resection after local recurrence following local excision[33]. Lower quality mesorectal excision leads to higher local recurrence and a reduction in survival, which therefore emphasizes the importance of patient selection for local excision in the first place.
Local excision following neoadjuvant therapy
With increasing interest in watch-and-wait approach for complete clinical response following neoadjuvant chemoradiation, there has also been a trend towards local excision to evaluate and confirm mural pathologic response. Additionally, local excision is being utilized more commonly for patients whose tumors have been clinically downstaged but not responded completely. However it should be kept in mind that even with a complete pathologic response of primary tumor (ypT0), the risk of nodal positivity remains 3%-6%[34-36].
In 2016, Shin et al.[37] published retrospective data of 34 patients who had local excision following neoadjuvant chemoradiation. They included patients with only complete or near complete clinical response. A pathologic complete response was achieved in 56% of patients, 35% had T1 and 9% had T2 tumor in their final pathology. Only 2 patients developed recurrence (one local recurrence and one distant metastasis) during a 5-year follow-up period. In this study, all lesions were located in low rectum; 28 patients had TAE and 6 patients had TAMIS.
Lee et al.[30] published a wider range of patients with more advanced stage. They investigated the role of TAMIS for patients with T2/T3 N+ disease who received neoadjuvant chemoradiation and responded clinically with negative lymph nodes on post treatment imaging and a final tumor showing a small whitish scar and/or shallow ulcer on sigmoidoscopy. On final pathology, 18 patients showed a pathologic complete clinical response of primary tumor, whereas 11 patients still had T2/T3 tumor. All of these patients refused to undergo further surgery and nearly half of them (5 of 11 patients) developed local and/or distant recurrence during the median follow-up of 36 months.
Local excision for more advanced tumors
As of today, the gold standard treatment of T2-T4 lesions is TME due to high risk of lymph node metastasis. Local excision of T2-T4 lesions can be considered as an oncologically inferior but less invasive alternative to radical excision in several clinical scenarios; including patients with multiple comorbidities who are medically unfit to undergo a major abdominopelvic procedure or patients with metastatic disease but who need local control for palliation [Figure 4]. There may also be some patients who demonstrate understanding and refuse radical resection to avoid its complications like fecal or sexual dysfunction, or to maintain intestinal continuity and avoid a permanent colostomy with an acceptable risk of higher recurrence rate.
Figure 4. An obstructing T3 tumor excised by transanal minimally invasive surgery for palliation in a patient with liver and lung metastases
Knowledge about the role of local excision for lesions deeper than T1 is mostly limited to small series of patients who declined TME or were unfit for abdominal surgery[19][Figure 5]. These series have reported significantly higher local recurrence rates. These series also do not follow a standard protocol of chemotherapy or radiotherapy.
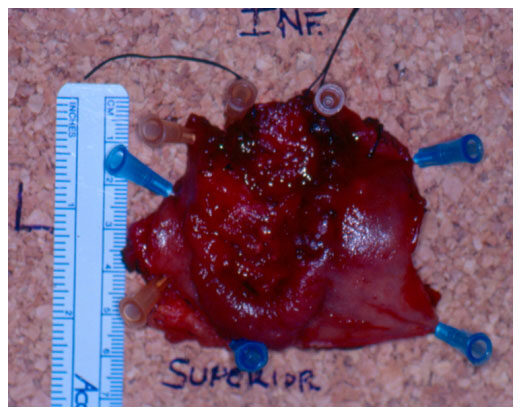
Figure 5. A T2 rectal tumor specimen fixed on board after transanal minimally invasive surgery excision
Lezoche et al.[38] studied the long-term outcomes of 70 patients with T2N0 rectal cancer randomized to TEM or laparoscopic TME. All patients in each group had received neoadjuvant chemoradiation. They reported a local recurrence rate of 5.7% after local excision versus 2.8% percent after laparoscopic TME, with a median follow-up of 84 months. There was no difference in disease-free survival.
Similarly, American College of Surgeons Oncology Group (ACOSOG) Z6041 trial investigated the role of local excision for clinically staged T2N0 rectal cancer following neoadjuvant chemoradiation. Local recurrence rate was reported as 4% and distant metastasis as 6% after a median follow-up of 56 months[39].
Functional outcomes and quality of life
The biggest driving force behind less invasive approaches for rectal cancer - especially for early rectal cancer - is the high morbidity of gold standard technique; TME. Functionally unfavourable outcomes in intestinal, urinary and sexual functions are the major concerns for patients’ postoperative quality of life (QoL). Local excision can potentially decrease the incidence of these complications and improve QoL. TEM has been associated with fecal incontinence due to its 4-cm diameter scope and rates of up to 37% worse incontinence has been reported after TEM[40]. TAMIS seems more advantageous in this regard as it utilizes a flexible port to access rectum. Although longer term follow-up data is available for TEM, the impact of TAMIS on patients’ QoL is unclear - due to it being a relatively new technique.
Recently, Clermonts et al.[41] published their data of 37 patients who underwent TAMIS for dysplastic sessile polyps or cT1 rectal cancer. They compared Short-Form 36 Health Survey responses of 37 patients to a healthy case-matched population. This study demonstrated that patients scored worse than healthy control group in physical functioning, general health perception and social functioning domains. After a 3-year follow-up, 9 patients reported improved fecal incontinence severity index (FISI) scores, 19 patients deteriorated and 9 patients remained same.
On the other hand, Verseveld et al.[42] compared QoL of 24 patients undergoing TAMIS before and 6 months after the surgery. They did not note any deterioration, but a general improvement in QoL and more specifically in FISI scores after TAMIS.
Schiphorst et al.[43] similarly reported FISI scores of 35 patients pre- and post-TAMIS. Their data showed that 3 of 18 patients with normal continence developed soiling after the surgery but 2 of them returned to normal in 6 months. Fifteen of 17 patients with abnormal FISI score showed improvement in fecal continence following TAMIS.
These studies yield varying results. It should be kept in mind that one of these studies compared TAMIS patients to healthy control group, and the other two compared the same patients’ pre- and post-operative status. A study comparing functional outcomes and QoL of patients who underwent TAMIS versus TME would give a more meaningful picture in terms of the functional benefits of local excision over radical resection.
Complications
Lee et al.[29] reported postoperative morbidity of TAMIS to be 9% in a series of 228 patients comprising both benign and malignant tumors. In another study by the same group, complication rates of TAMIS in a group of 110 rectal cancer patients was 15%. The most common complications of TAMIS were urinary retention, perioperative bleeding and peritoneal violation; similar to TAE and TEM[30].
Both urinary retention and peritoneal entry have been associated with anterior and lateral location of the tumor. Urinary retention is usually self-limited and treated by temporary urinary catheterization. Entry into peritoneal cavity has been reported more frequently with upper lesion location (> 8-10 cm from anal verge). The largest TAMIS series published to date had an incidence of peritoneal entry of only 2%. In contrast, the incidence is reported as 6%-8.6% for TEM. Either transanal or laparoscopic suturing can be utilized when a peritoneal defect occurs. It is not associated with increased morbidity. Risk is further reduced in the setting of preoperative bowel preparation and intravenous antibiotic treatment for 24 h postoperatively[18,19,44]. In these patients, a gastrograffin enema is recommended on postoperative day 3 to document the absence of a leak[2].
Mean blood loss for local excision of rectal cancer is 28 mL[30]. Increased bleeding has been associated with large tumor size. Cases of post-procedural hemorrhage that do not stop spontaneously have in all cases been managed successfully either endoscopically or with examination under anesthesia and sewing[1].
Less commonly reported complications include urinary tract infection, subcutaneous emphysema, scrotal edema and hemorrhoidal thrombosis[2].
Barendse et al.[45] concluded that a learning curve effect was observed in complication rates. However, a specific case volume was not defined in this study and complication rates before and after the learning curve were not compared. Lee et al.[46] investigated the learning curve in a single high volume tertiary care referral center and found that the learning curve of TAMIS for rectal neoplasms is 14-24 cases. This study did not show a difference in complication rates before and after the learning curve.
Conclusion
Based on currently available clinical data, TAMIS in experienced hands, results in the high quality local excision of early rectal tumors with low histological margin positivity in an efficient manner and low recurrence rates in context of favourable histologic properties with an excellent morbidity profile with no long term adverse effect on continence. The role of TAMIS for more advanced tumors and in the post-neoadjuvant setting needs clarification by further studies. It can also be offered as a palliative procedure to patients with metastatic disease, which would potentially avoid complications of a major surgery. TAMIS has enabled the performance of high quality local excision of rectal lesions by many colorectal surgeons, integrating transanal endoscopic surgery into mainstream practice. Currently surgeon preference and device availability govern which platform is selected for use. As with all new techniques used in the management of neoplastic disease, appropriate training must be ensured and the continued assessment and assurance of oncological outcome - via databases - must be maintained.
Declarations
Authors’ contributionsDesign, literature research, manuscript writing, manuscript editing, manuscript revision: Erkan A, Kelly JJ, Monson JRT
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2018.
REFERENCES
2. Plummer JM, Leake PA, Albert MR. Recent advances in the management of rectal cancer: no surgery, minimal surgery or minimally invasive surgery. World J Gastrointest Surg 2017;9:139-48.
3. Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 2010;24:2200-5.
4. Atallah SB, Larach S, deBeche-Adams TC, Albert MR. Transanal minimally invasive surgery (TAMIS): a technique that can be used for retrograde proctectomy. Dis Colon Rectum 2013;56:931.
5. Brunner W, Rossetti A, Vines LC, Kalak N, Bischofberger SA. Anastomotic leakage after laparoscopic single-port sigmoid resection: combined transanal and transabdominal minimal invasive management. Surg Endosc 2015;29:3803-5.
6. Caycedo-Marulanda A, Jiang HY, Kohtakangas EL. Transanal minimally invasive surgery for benign large rectal polyps and early malignant rectal cancers: experience and outcomes from the first Canadian centre to adopt the technique. Can J Surg 2017;60:416-23.
7. deBeche-Adams T, Nassif G. Transanal minimally invasive surgery. Clin Colon Rectal Surg 2015;28:176-80.
8. Hayashi K, Kotake M, Kakiuchi D, Yamada S, Hada M, Kato Y, Hiranuma C, Oyama K, Hara T. Laparoscopic total pelvic exenteration using transanal minimal invasive surgery technique with en bloc bilateral lymph node dissection for advanced rectal cancer. Surg Case Rep 2016;2:74.
9. Quaresima S, Balla A, Franceschilli L, La Torre M, Iafrate C, Shalaby M, Di Lorenzo N, Sileri P. Transanal minimally invasive surgery for rectal lesions. JSLS 2016; doi: 10.4293/JSLS.2016.00032.
10. Morson BC, Bussey HJ, Samoorian S. Policy of local excision for early cancer of the colorectum. Gut 1977;18:1045-50.
11. Garcia-Aguilar J, Mellgren A, Sirivongs P, Buie D, Madoff RD, Rothenberger DA. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg 2000;231:345-51.
12. Paty PB, Nash GM, Baron P, Zakowski M, Minsky BD, Blumberg D, Nathanson DR, Guillem JG, Enker WE, Cohen AM, Wong WD. Long-term results of local excision for rectal cancer. Ann Surg 2002;236:522-29.
13. Salehomoum NM, Nogueras JJ. Conventional transanal excision: current status and role in the era of transanal endoscopic surgery. Semin Colon Rectal Surg 2015;26:6-8.
14. Buess G, Hutterer F, Theiss J, Böbel M, Isselhard W, Pichlmaier H. A system for a transanal endoscopic rectum operation. Chirurg 1984;55:677-80.
15. Rai V, Mishra N. Transanal approach to rectal polyps and cancer. Clin Colon Rectal Surg 2016;29:65-70.
16. Maglio R, Muzi GM, Massimo MM, Masoni L. Transanal minimally invasive surgery (TAMIS): new treatment for early rectal cancer and large rectal polyps-experience of an Italian center. Am Surg 2015;81:273-7.
17. Althumairi AA, Gearhart SL. Local excision for early rectal cancer: transanal endoscopic microsurgery and beyond. J Gastrointest Oncol 2015;6:296-306.
18. Melin AA, Kalaskar S, Taylor L, Thompson JS, Ternent C, Langenfeld SJ. Transanal endoscopic microsurgery and transanal minimally invasive surgery: is one technique superior? Am J Surg 2016;212:1063-7.
19. Thompson EV, Bleier JI. Transanal minimally invasive surgery. Clin Colon Rectal Surg 2017;30:112-9.
20. Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CA. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol 2005;23:6199-206.
21. McLemore EC, Weston LA, Coker AM, Jacobsen GR, Talamini MA, Horgan S, Ramamoorthy SL. Transanal minimally invasive surgery for benign and malignant rectal neoplasia. Am J Surg 2014;208:372-81.
22. Loughrey MB, Quirke P, Shepherd NA. Standards and datasets for reporting cancers. 4th ed. London: the Royal College of Pathologists; 2017.
23. Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, Fujiyoshi T, Uchida Y. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 1995;38:1286-95.
24. Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, Iwashita A, Ajioka Y, Watanabe H, Watanabe T, Muto T, Nagasako K. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 2004;39:534-43.
25. Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 1993;25:455-61.
26. Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002;45:200-6.
27. Monson JR, Weiser MR, Buie WD, Chang GJ, Rafferty JF, Buie WD, Rafferty J; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of rectal cancer (revised). Dis Colon Rectum 2013;56:535-50.
28. Brunner W, Widmann B, Marti L, Tarantino I, Schmied BM, Warschkow R. Predictors for regional lymph node metastasis in T1 rectal cancer: a population-based SEER analysis. Surg Endosc 2016;30:4405-15.
29. Lee L, Edwards K, Hunter IA, Hartley JE, Atallah SB, Albert MR, Hill J, Monson JR. Quality of local excision for rectal neoplasms using transanal endoscopic microsurgery versus transanal minimally invasive surgery: a multi-institutional matched analysis. Dis Colon Rectum 2017;60:928-35.
30. Lee L, Burke JP, deBeche-Adams T, Nassif G, Martin-Perez B, Monson JRT, Albert MR, Atallah SB. Transanal minimally invasive surgery for local excision of benign and malignant rectal neoplasia: outcomes from 200 consecutive cases with midterm follow up. Ann Surg 2018;267:910-6.
31. Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah S. A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol 2014;18:775-88.
32. Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis 2013;15:788-97.
34. Bedrosian I, Rodriguez-Bigas MA, Feig B, Hunt KK, Ellis L, Curley SA, Vauthey JN, Delclos M, Crane C, Janjan N, Skibber JM. Predicting the node-negative mesorectum after preoperative chemoradiation for locally advanced rectal carcinoma. J Gastrointest Surg 2004;8:56-62.
35. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Kepka L, Winkler-Spytkowska B, Suwiński R, Oledzki J, Stryczyńska G, Wieczorek A, Serkies K, Rogowska D, Tokar P; Polish Colorectal Study Group. Prediction of mesorectal nodal metastases after chemoradiation for rectal cancer: results of a randomised trial: implication for subsequent local excision. Radiother Oncol 2005;76:234-40.
36. Yeo SG, Kim DY, Kim TH, Chang HJ, Oh JH, Park W, Choi DH, Nam H, Kim JS, Cho MJ, Kim JH, Park JH, Kang MK, Koom WS, Kim JS, Nam TK, Chie EK, Kim JS, Lee KJ. Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09-01). Ann Surg 2010;252:998-1004.
37. Shin YS, Yoon YS, Lim SB, Yu CS, Kim TW, Chang HM, Park JH, Ahn SD, Lee SW, Choi EK, Kim JC, Kim JH. Preoperative chemoradiotherapy followed by local excision in clinical T2N0 rectal cancer. Radiat Oncol J 2016;34:177-85.
38. Lezoche E, Guerrieri M, Paganini AM, Feliciotti F. Long-term results of patients with pT2 rectal cancer treated with radiotherapy and transanal endoscopic microsurgical excision. World J Surg 2002;26:1170-4.
39. Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, Thomas CR Jr, Chan E, Cataldo PA, Marcet JE, Medich DS, Johnson CS, Oommen SC, Wolff BG, Pigazzi A, McNevin SM, Pons RK, Bleday R. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 2015;16:1537-46.
40. Dafnis G, Påhlman L, Raab Y, Gustafsson UM, Graf W. Transanal endoscopic microsurgery: clinical and functional results. Colorectal Dis 2004;6:336-42.
41. Clermonts SHEM, van Loon YT, Wasowicz DK, Langenhoff BS, Zimmerman DDE. Comparative quality of life in patients following transanal minimally invasive surgery and healthy control subjects. J Gastrointest Surg 2018;22:1089-97.
42. Verseveld M, Barendse RM, Gosselink MP, Verhoef C, de Graaf EJ, Doornebosch PG. Transanal minimally invasive surgery: impact on quality of life and functional outcome. Surg Endosc 2016;30:1184-7.
43. Schiphorst AH, Langenhoff BS, Maring J, Pronk A, Zimmerman DD. Transanal minimally invasive surgery: initial experience and short-term functional results. Dis Colon Rectum 2014;57:927-32.
44. Keller DS, Haas EM. Transanal minimally invasive surgery: state of the art. J Gastrointest Surg 2016;20:463-9.
45. Barendse RM, Dijkgraaf MG, Rolf UR, Bijnen AB, Consten EC, Hoff C, Dekker E, Fockens P, Bemelman WA, de Graaf EJ. Colorectal surgeons' learning curve of transanal endoscopic microsurgery. Surg Endosc 2013;27:3591-602.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Erkan A, Kelly JJ, Monson JRT. Current state of transanal minimally invasive surgery in the management of rectal cancer. Mini-invasive Surg 2018;2:30. http://dx.doi.org/10.20517/2574-1225.2018.51
AMA Style
Erkan A, Kelly JJ, Monson JRT. Current state of transanal minimally invasive surgery in the management of rectal cancer. Mini-invasive Surgery. 2018; 2: 30. http://dx.doi.org/10.20517/2574-1225.2018.51
Chicago/Turabian Style
Erkan, Arman, Justin J. Kelly, John R. T. Monson. 2018. "Current state of transanal minimally invasive surgery in the management of rectal cancer" Mini-invasive Surgery. 2: 30. http://dx.doi.org/10.20517/2574-1225.2018.51
ACS Style
Erkan, A.; Kelly JJ.; Monson JRT. Current state of transanal minimally invasive surgery in the management of rectal cancer. Mini-invasive. Surg. 2018, 2, 30. http://dx.doi.org/10.20517/2574-1225.2018.51
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 10 clicks
Cite This Article 10 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.