Imaging
Abstract
The incidence of renal cell carcinoma is rising and its represents the 2%, 3% of all cancers. The increased use of ultrasonography, contrast enhanced ultrasonography, computed tomography and magnetic resonance imaging have resulted in incidentally detected small renal masses (SRMs). SRMs represent a heterogeneous group of tumors that included metastatic lesions, benign, malignant, and cystic lesions. With the increase number of renal incidentalomas, we have seen an increase in therapeutic choices (surgery, ablation therapies and active surveillance). The role of imaging has progressively grown over the decades and became currently a cornerstone that is needed to perform diagnosis, treatment and follow-up of SRMs after ablation treatment. Hence, in this review, we critically assess recent literature on the role of imaging in the context of ablation management of SRMs with a focus on the diagnosis and follow-up protocol.
Keywords
Introduction
During the last decades, there has been an increase in the incidence of small renal masses (SRMs). This is likely because of increased use of axial and abdominal imaging and longer life expectancy[1].
SRMs are defined as renal masses with a maximum diameter of less than 4 cm and represent an extremely heterogeneous category of lesions including benign, malignant, solid or cystic ones. Though SRMs in 80% of cases are malignant tumors, they show a good natural history according to DISSRM Registry. Indeed, SRMs growth rate and variability decrease with time during active surveillance: in patients of advanced age or with serious comorbid conditions, SMRs can be managed conservatively with little change in overall survival or cancer-specific survival rate[2]. The treatment paradigm historically centered around surgery, from open one to minimally invasive surgery, such as enucleation or enucleoresection, in order to preserve as much as possible healthy renal parenchyma, reducing the risk of chronic kidney disease (CKD)[3]. However, thanks to more modern technologies, the concept of active surveillance has grown rapidly and nowadays in selected patients, we could offer a standardized protocol of surveillance or a focal management strategy. In addition, renal tumor ablation (focal management) has been developed rapidly during the last 2 decades and currently could be a real alternative option in the opportunely selected candidate to obtain local tumor control, functional preservation, fewer complications and a shorter recovery in comparison to surgery[2,3].
The imaging in the context of ablation therapy for SRMs is a cornerstone that is needed in all the pathway schedules: from diagnosis to treatment and eventually for surveillance and follow-up protocols. Hence, in this review, we critically assess recent literature on the role of imaging in the context of minimally invasive management of SRMs, focusing in particular on diagnosis and follow-up after ablative treatment.
Imaging modalities - diagnosis
The improvement and wide spreading of ever more efficient imaging techniques have leaded in the last years to a sensible increase in the diagnosis of incidental renal masses (IRM). Different imaging modalities are used: (1) ultrasonography (US); (2) contrast enhancement ultrasonography (CEUS); (3) computed tomography (CT); and (4) magnetic resonance imaging (MRI).
US
In every day clinical practice, US is the first imaging technique that allows the discovery of IRM. Generally, after having been discovered, IRM are better categorized and staged by using CT scan or abdominal MRI. The choice is extremely related to the comorbidity of the patients and the presence of a CKD and the experience of the radiologist.
According to Oh et al.[4], US has the sensitivity (SE), specificity (SP), positive predictive value (PPV), negative predictive value (NPV) and accuracy for renal cell carcinoma (RCC) diagnostic efficacy of 60.5%, 72.7%, 34.8%, 63.6% respectively.
The advange of US included that the target of examination (SMRs) is clearly visible. All the conditions that limit the US examination, such as obesity or meteorism, represent limits also for CEUS.
CEUS
Otherwise, CEUS has a good potential in the categorization of focal SRMs and has had an increasing use and widespread adoption worldwide due to its advantages in the evaluation of enhancement pattern of the renal lesion without the risk of nephrotoxicity and the lack of ionizing radiation. The contrast agent is made of microbubbles and has no toxic effects on renal parenchyma. As concerning CEUS, PPV, SE, SP, NPV are 86.8%, 63.6%, 89.2%, 58.3% and 81.6% respectively, for RCC diagnostic efficacy[4]. CEUS advantages include safety, patient tolerance, real-time imaging capability, and costs[5].
Hence, CEUS could be a useful instrument in the pre-operatively settings for characterization and diagnosis of SRMs but also in the follow-up, due for its strength in the detection of acute complication and early intralesional enhancement[6].
Another use of CEUS is to distinguish kidney tumors and/or other conditions in which the convective B mode and US Doppler have not helpful the radiologist to a definitive diagnosis: for example, the pseudotumors have the same degree of enhancement as the remaining healthy renal parenchyma[7,8].
Particularly CEUS seems to be useful in some mimicking anatomical variations: pseudotumors, for example, have the same enhancing characteristics of the surrounding parenchyma [Figure 1] in all phases[9,10]. Conversely, in the majority of cases, the enhancement in kidney tumors is different from the nearly parenchyma: at least one vascular phase has a variation in the degree or distribution of enhancement. CEUS is also appropriate in the analysis and characterization of complex kidney cysts and particularly in the differentiation of Bosniak classification of renal cysts [Figures 2-5].
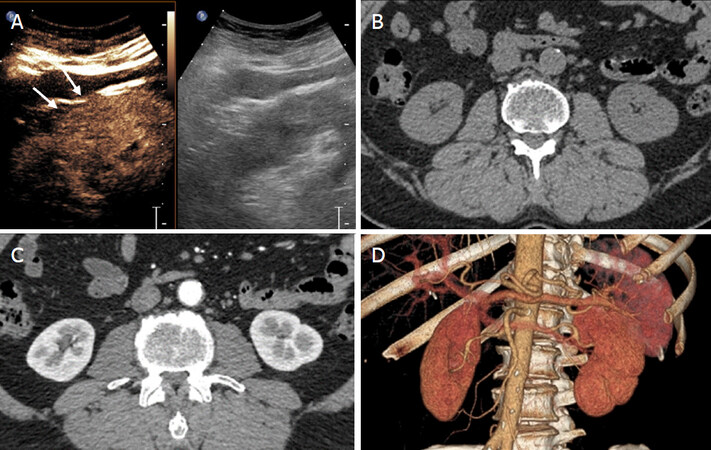
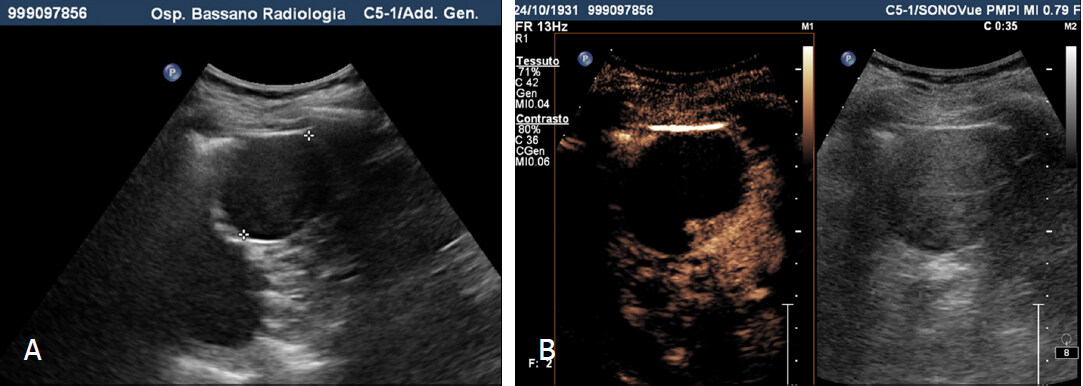
Figure 1. Pseudotumor. A: Grey-scale sonography shows isoechoic cortical mass (or dromedary hump?) in the midportion of the kidney (arrows); on contrast-enhanced sonograms the lesion shows enhancement equal (or similar) to the kidney in all vascular phases. Computed tomography with contrast injection before (B), after (C) and 3D reconstruction (D) doesn’t show focal renal lesions
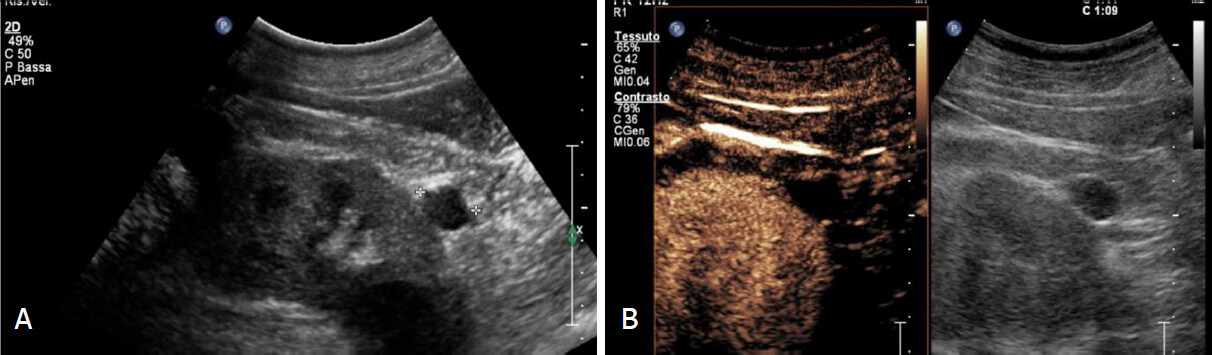
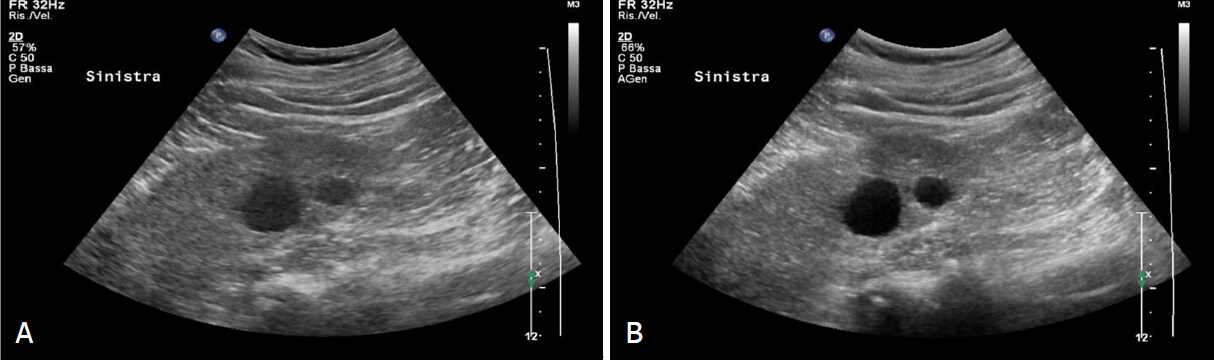
Figure 2. Bosniak 2 cyst. A: Grey scale and contrast enhanced sonograms shows small exophitic hypoechoic mass with smooth margin in lower pole; B: Completely avascular after microbubble injection
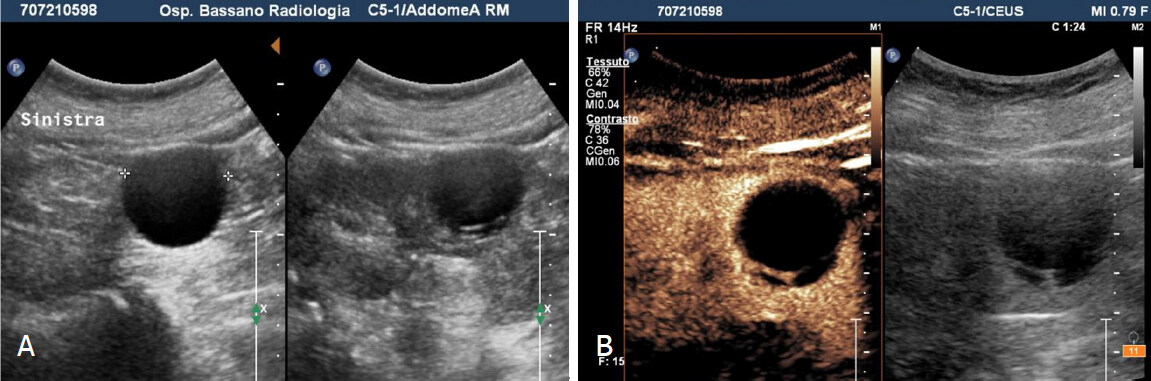
Figure 3. Bosniak 2F cyst. A: Grey scale sonography shows in the mid portion of the left kidney cystic lesion with thin hyperechoic septum; B: Contrast enhanced sonography reveal homogeneous enhancement of the septum without parietal nodule
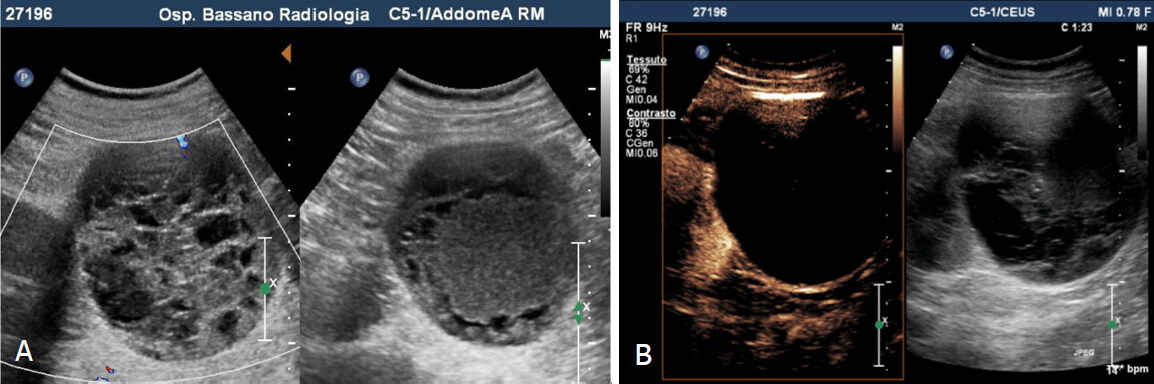
Figure 4. Bosniak 3 cyst. A: Greyscale sonography shows voluminous mass with mixed echo structure (anechoic and hyperechoic components); B: Contrast enhanced sonography reveals cystic lesion characterized by irregular, thickened and vascularized walls
Figure 5. Bosniak 4 cyst. A: Grey scale shows hypoechoic round lesion with smooth margin; B: Contrast- enhanced sonography reveals a cystic lesion with mural nodules characterized by strong enhancement
Compared with CT, CEUS seems to have the higher preciseness of CT for the characterization of renal cystic lesions according to lesional enhancement (LE). Infact, in some cases, CT and/or RMI can’t recognize LE with inconclusive findings[11-13]. CEUS has a greater SP in characterizing renal cystic lesions, in particular the presence and thickness of a septum and/or the presence of a solid intracystic components[14-16].
Anyway, it is well-known the role of CT in the malignant cystic lesion for staging: now the CEUS is useful in the follow up of complex cystic lesions that are not suitable for surgery and in the future it could replace the CT[15].
CEUS plays an important role during the follow up: it avoids unnecessary studies with CT. The advantages of CEUS are considerable: a conventional US B-mode diagnosis in most cases a simple cyst [Figure 6], but CEUS detects neoangiogenesis (blood flow in hypovascularized lesions) with greater accuracy[16]. It allows a clearer distinction between solid and complex cystic lesions, above all it helps to characterize non-diagnostic lesions with other imaging (CT, US, B-mode)[17]. In agreement with Bertolotto et al.[18], CEUS allows a better identification of the lesions, expecially in the case of small or multifocal lesions, with the help of the image fusion system.
CT
CT exposes the subject to a relatively high dose of radiation, and the iodinated contrast agents have a potential risk of nephrotoxicity in patients with renal impairment. Furthermore, on CT examination, enhancement is defined when an increase in attenuation > 15 Hounsfield Units is observed between the unenhanced and enhanced phases[19].
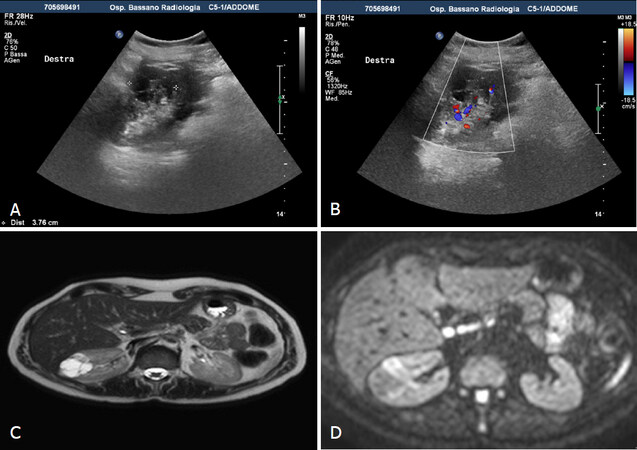
CT is the gold standard in the worldwide for staging renal tumors and SRMs. However, in clinical practice, most CT scan studies are not performed with a specific renal protocol: most of time protocol are inadequate to characterize the lesions. Hence, indeterminate renal lesions are frequently identified [Figure 7]. In addition, during follow-up US assessment should be comprehensive, including CEUS, to obviate an unnecessary CT study[16].
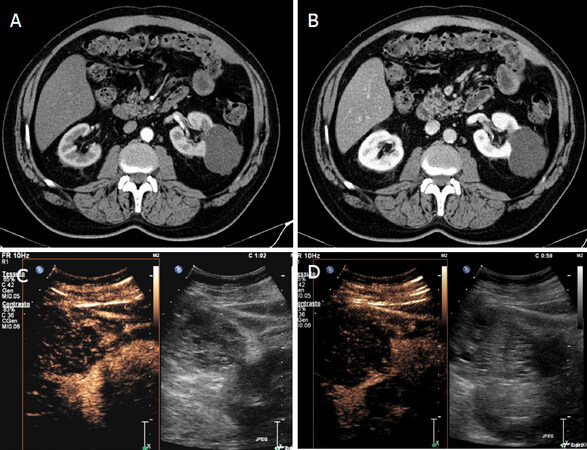
Figure 7. Indeterminate renal lesions at CT study. Contrast CT shows in the left kidney voluminous, partially exophitic, hypodense lesion with irregular margins and without enhancement in phases arterial (A) and venous phase (B). Greyscale and contrast-enhanced sonography (C-D) shows a lesion with mixed echo structure and after microbubble injection shows multiple vascularized septa (Bosniak 4)
MRI
MRI is considered the most accurate diagnostic tool, with SE and SP in characterizing SMRs ranging from 88% to 100% and from 83% to 93%, respectively[6]. However, it is also the most expensive imaging technique[6] and cannot be used in patients with a pacemaker, uncooperative patients, and patients with severe renal failure. There are occasionally issues related to patient tolerance and safety, in particular the risk of nephrogenic systemic fibrosis in patients with estimated glomerular filtration rates under 30 mL/min, when exposed to MRI contrast agents[7-9]. Moreover, the MRI characterization of SMRs may be difficult, as image subtraction cannot be effectively performed.
MRI indication is used both to evaluate renal masses of nature to be and determined to stage RCC, considering that MRI is more specific than CT scan for benign lesions [Figure 8][19,20].
Figure 8. Fusion-guided microwave ablation. 75 years old patient with exophitic in the right kidney, identified by sonography and contrast-enhanced ultrasound (A) and confirmed by MR (B), it was a RCC (biopsy proven) treated with microwaves through guided fusion technique (C-D). RCC: renal cell carcinoma
Moreover, MRI helps to classify renal tumors according to specific characteristics (solid vs. cystic), expecially in cases of doubtful CT[18]. The most recent MRI schemes to study SMRs, use different parameters, such as DWI and dynamic contrast-enhanced imaging, in order to obtain a complete and precise lesion characterization[21][Figure 9].
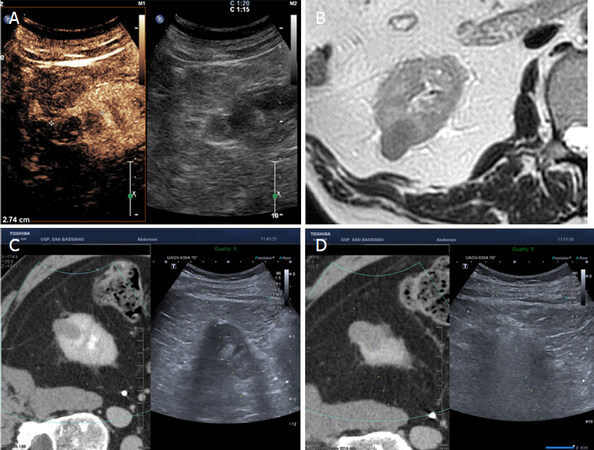
Figure 9. MRI with DWI. Greyscale sonography shows right midportion complex cystic, without vascularization at color-Doppler analysis (A, B). MR reveals on T2 weighted images complex cystic lesion (Bosniak III-IV) with multiple thin septa and hypointense intramural nodule (C). DWI weighted images show restriction of the intramural nodule: it was a papillary RCC (D). DWI: diffusion weighted imaging; RCC: renal cell carcinoma
Hence, using those parameters could be helpful to differentiate benign from malignant SRMs, but also have a role in distinguishing the different subtypes of RCC and tumor’s grade[22,23].
Imaging modalities - follow-up
The aim of ablation therapy of SRMs is to obtain a complete necrosis of the lesion with an obligatory damage to adjacent healthy renal tissue to achieve a negative tumor margin and decrease the liability of residual disease near the ablation zone. The absence of contrast enhancement at the site of treatment, indicates success after procedure. The importance of imaging is both to evaluate technique success and to follow up the ablated mass.
Historically there has been different debate regarding the definitions of recurrence after ablation for SRMs. The literature has a not unique definition of recurrence after treatment for RCC and particularly after ablation there are different controversy also in the definition of progression, treatment success, and treatment efficacy. As opposed to surgery, the ablation efficacy is based typically on radiological findings[24].
Literature lacks of good quality data about long-term efficacy of percutaneous ablation treatment for SRMs. Regarding radiofrequency ablation several studies report short-term outcomes. In a limited period of follow up: (1) tumors with a diameter < 4 cm have a low probability of residual disease after a single procedure[25,26]; (2) central located tumors have lower rates of residual disease when compared with exophytic tumors[27]; and (3) tumors with a diameter > 3 cm compared with smaller tumors, have an higher rates of residual disease after the first treatment[27].
Anyway, follow-up can be performed by CEUS, CT or MRI. CT and MRI are the best options for the patient because of their high resolution and chance to detect recurrences. However, as for the diagnostic pathway, the cumulative radiation dose could be very high. Another problem is that the CT scan requires iodine contrast, which is a well-known nephrotoxic substance.
The current guidelines don’t explain the tyme and duration imaging follow up after ablation treatment. The AUA recommends cross-sectional imaging at 3 months and 6 months after ablation, then annually for 5 years. After ablation treatments, the timing of initial follow up varies according to operator’s preference; the range is from day 0 to 1 month post operatively[28-30]. Generally, the majority of residual tumor is detected within the first 3 months after ablation, the urge for early short interval follow-up. According to Matin et al.[26], the most frequent timing of recidive is within the first 3 months after treatment: this is important in order to programme an early follow up.
CEUS is increasingly used during follow-up and particularly on close postoperative time: day 1 to 1 month. The main aim is looking complications.
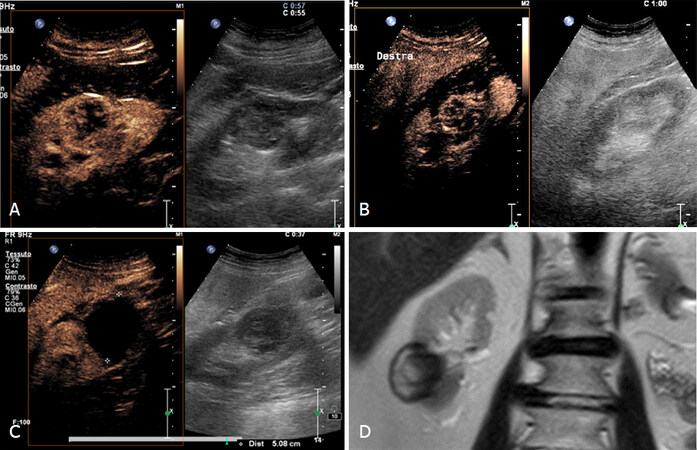
The first imaging sign of tumor persistence after ablation treatment, is the persistence of vascularization in enhancement to imaging. This signal is the first post operative day not allow to diagnose a failure of the procedure. For this reason, it is important to perform a CEUS at 30 days to evaluate a successful or uncessful ablation [Figure 10][8].
Figure 10. Follow-up after cryoablation. A: 84 years old patient with 5 cm RCC; B: Postoperative day 1 and enhancement; C: no tumor persistence and often a repeated CEUS after 1 month is recommended in lesions that maintain vascularity on postoperative to exclude residual tumor; D: MR after 6 months confirms the success of the cryoablation treatment. CEUS: contrast enhancement ultrasonography; RCC: renal cell carcinoma
This is particularly true in cryoablated lesions (15%-20% of cases) that can exhibit post-ablation contrast enhancement on initial postoperative imaging and also for the first few months, which resolves in 45% of patients in a period from 1 up to 14 months. Most recurrent lesions develop after the first year therefore, different authors suggest surveillance protocol based on CT or MRI with short interval for the first year (1, 3, 6, and 12 months respectively after treatment), and then every 6 months for the second year and then annually thereafter[31].
Discussion
The role of imaging in the diagnosis and decision-making of management of SMRs has been crucial over the last years. Firsty, the development of new technology and the acquisition of extremely sophisticated machines has allowed a better quality and better precision in the characterization and diagnosis of SRMs. Hence, the quality in the diagnosis allowed a better decision-making process, tailored for each patients. NSS is performed for SMRs, but as many 30% of the lesions prove benign nature; for this reasons, there is increased interest in preoperative study of SMRs subtypes[27]. Secondary the imaging modalities have changed the treatment process itself: ablation modalities are performed nowadays often via imaging-guide mode and in 2017 AUA Guidelines has recommended a percutaneous approach as the best choice if possible, in the ablation treatment of SRMs[28]. Percutaneous biopsy results were shown to be discordant with surgical pathology from resected specimens in 8% of cases: is a safe procedure, Hemorrhage, if it occurs, is usually subclinical and needle tract seeding is exceedingly rare. A renal tumor biopsy before ablative therapy and systemic therapy without previous pathology must be considered in select patients who are considered for AS, in order to obtaining a histological diagnosis preoperative[32]. Third, the success of a treatment of a kidney tumor is not only in the diagnosis and management but also in the follow-up of that lesions. For this reason, the real success of ablation therapy is evaluated by routinely imaging follow up. The surveillance with CT and/or MRI is crucial and indispensable for determining initial treatment success, detecting recurrence disease at the ablation site, or new metachronous lesions. The introduction of multidisciplinary team with urologists and radiologists could improve the follow up after ablation therapy[33]. As concerning the first diagnosis, frequently pathologic features are observed in RCC like hemorrhage, necrosis and cystic change. CT scan correlates these features with the existence of necrotic degeneration or intratumoral cystis: the washout and LE pattern of CEUS might relate to the pathologic features of RCC such as intratumoral blood flow, thin-walled blood vessels and hemorrhage. CEUS could be used for US diagnosis of pseudocapsules like a useful sign both in the differential diagnosis of RCC and in the choice of NSS[34].
Conclusion
The ablation procedure for SRMs is increasing worldwide together with an evolution of imaging assessment. Multiple combination of different technology and modalities is offering different opportunity for a more precisely diagnosis of SRMs. Furthermore, this real cutting-edge imaging protocols might be more effective and crucial in the patient management. However, there is a need of more precisely evidence-based algorithms for diagnostic and treatment pathway that could be the glue that integrate the imaging for diagnosis with the imaging for treatment.
Declarations
Authors’ contributionsMade contributions to conception and design of the study/review and performed data analysis and interpretation: Cicero C, Casarin A
Performed data acquisition: Currò F, Campo I
Made substantial contributions to conception and design of the study/review, performed an entire revision of data analysis, re-interpretation and the revision for editors: Silvestri T, Bada M
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2019.
REFERENCES
1. Kane CJ, Mallin K, Ritchey J, Cooperberg MR, Carroll PR. Renal cell cancer stage migration: analysis of the National Cancer Data Base. Cancer 2008;113:78-83.
2. Uzosike AC, Patel HD, Alam R, Schwen ZR, Gupta M, et al. Growth Kinetics of Small Renal Masses on Active Surveillance:Variability and Results from the DISSRM Registry. J Urol 2018;199:641-8.
3. Bertolotto M, Catalano O. Contrast-enhanced ultrasound: past, present, and future. Ultrasound Clin 2009;4:339-67.
4. Oh TH, Lee YH, Seo IY. Diagnostic efficacy of contrast-enhanced ultrasound for small renal masses. Korean Journal of Urology. Diagnostic Efficacy of Contrast-Enhanced Ultrasound for Small Renal Masses. Korean J Urol 2014;55:587-92.
5. Bertolotto M, Derchi LE, Cicero C, Iannelli M. Renal Masses as Characterized by Ultrasound Contrast. Ultrasound Clin 2013;8:581-92.
6. Barozzi L, Valentino M, Bertolotto M, Pavlica P. Contrast enhanced ultrasound of renal diseases. Arch Ital Urol Androl 2010;82:232-7.
7. Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017. Ultraschall Med 2018;39:e2-44.
8. Bertolotto M, Siracusano S, Cicero C, Iannelli M, Silvestri T, et al. Cryotherapy of Renal Lesions: Enhancement on Contrast-Enhanced Sonography on Postoperative Day 1 Does Not Imply Viable Tissue Persistence. J Ultrasound Med 2017;36:301-10.
9. Correas JM, Claudon M, Tranquart F, Hélénon AO. The kidney: imaging with microbubble contrast agents. Ultrasound Q 2006;22:53-66.
10. Mazziotti S, Zimbaro F, Pandolfo A, Racchiusa S, Settineri N, et al. Usefulness of contrast-en-hanced ultrasonography in the diagnosis of renal pseudotumors. Abdom Imaging 2010;35:241-5.
11. Barr RG, Peterson C, Hindi A. Evaluation of indeterminate renal massess with contrast-enhanced US: a diagnostic performance study. Radiology 2014;271:133-42.
12. Sanz E, Hevia V, Gomez V, Álvarez S, Fabuel JJ, et al. Renal complex cystic masses: usefulness of contrast-enhanced ultrasound (CEUS) in their assessment and its agreement with computed tomography. Curr Urol Rep 2016;17:89.
13. Quaia E, Bertolotto M, Cioffi V, Rossi A, Baratella E, et al. Comparison of contrast-enhanced sonography with unenhanced sonography and contrast-enhanced CT in the diagnosis of malignancy in complex renal masses. AJR Am J Roentgenol 2008;191:1239-49.
14. Clevert DA, Minaifar N, Weckbach S, Jung EM, Stock K, et al. Multislice computed tomography versus contrast-enhanced ultrasound in evaluation of complex cystic renal masses using the Bosniak classification system. Clin Hemorheol Micro 2008;39:171-8.
15. Park BK, Kim B, Kim SH, Ko K, Lee HM, et al. Assessment of cystic renal masses based on Bosniak classification: comparison of CT and contrast-enhanced US. Eur J Radiol 2007;61:310-4.
16. Harkanyi Z. Potential applications of contrast-enhanced ultrasound in pediatric patients. Ultrasound Clin North Am 2013;8:403-22.
17. Ascenti G, Mazziotti S, Zimbaro G, Settineri N, Magno C, et al. Complex Cystic Renal Masses: Characterization with Contrast-enhanced US. Radiology 2007;243:158-65.
18. Bertolotto M, Cicero C, Perrone R, Degrassi F, Cacciato F, et al. Renal masses with equivocal enhamcement at CT: characterization with contrast-enhanced ultrasound. AJR Am J Roentgenol 2015;205:W557-65.
19. Di Vece F, Tombesi P, Ermili F, Sartori S. Management of incidental renal masses: time to consider contrast-enhanced ultrasonography. Ultrasound 2016;24:34-40.
20. Helck A, D’Anastasi M, Notohamiprodjo M, Thieme S, Sommer W, et al. Multimodality imaging using ultrasound image fusion in renal lesions. Clin Hemorheol Micro 2012;50:79-89.
21. Heilbrun ME, Remer EM, Casalino DD, Beland MD, Bishoff JT, et al. ACR appropriateness criteria indeterminate renal mass. J Am Coll Radiol 2015;12:333-41.
22. Kreft BP, Muller-Miny H, Sommer T, Steudel A, Vahlensieck M, et al. Diagnostic value of MR imaging in comparison to CT in the detection and differential diagnosis of renal masses: ROC analysis. Eur Radiol 1997;7:542-7.
23. Pedrosa I, Chou MT, Ngo L, H Baroni R, Genega EM, et al. MR classification of renal masses with pathologic correlation. Eur Radiol 2008;18:365-75.
24. Pedrosa I, Alsop DC, Rofsky NM. Magnetic resonance imaging as a biomarker in renal cell carcinoma. Cancer 2009;115(10 Suppl):2334-45.
26. Matin SF, Ahrar K, Cadeddu JA, Gervais DA, McGovern FJ, et al. Residual and Recurrent Disease Following Renal Energy Ablative Therapy: A Multi-Institutional Study. J Urol 2006;176:1973-7.
27. Del Cura JL, Zabala R, Iriarte JI, Unda M. Treatment of renal tumors by percutaneous ultrasound-guided radiofrequency ablation using a multitined electrode: effectiveness and complications. Eur Urol 2010;57:459-65.
28. Farrell MA, Charboneau WJ, DiMarco DS, Chow GK, Zincke H, et al. Imaging-guided radiofrequency ablation of solid renal tumors. AJR Am J Roentgenol 2003;180:1509-13.
29. Ferakis N, Bouropoulos C, Granitsas T, Mylona S, Poulias I. Longterm results after computed-tomography-guided percutaneous radiofrequency ablation for small renal tumors. J Endourol 2010;24:1909-13.
30. Donat SM, Diaz M, Bishoff JT, Coleman JA, Dahm P, et al. Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol 2013;190:407-16.
31. Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol 2017;198:520-9.
32. Caoili EM, Davenport MS. Role of Percutaneous Needle Biopsy for Renal Masses. Semin Intervent Radiol 2014;31:20-6.
33. Rutherford EE, Cast J EI, Breen DJ. Immediate and long-term CT appearances following radiofrequency ablation of renal tumours. Clin Radiol 2008;63:220-30.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Cicero C, Casarin A, Currò F, Campo I, Bada M, Silvestri T. Imaging. Mini-invasive Surg 2019;3:25. http://dx.doi.org/10.20517/2574-1225.2018.012
AMA Style
Cicero C, Casarin A, Currò F, Campo I, Bada M, Silvestri T. Imaging. Mini-invasive Surgery. 2019; 3: 25. http://dx.doi.org/10.20517/2574-1225.2018.012
Chicago/Turabian Style
Cicero, Calogero, Andrea Casarin, Francesca Currò, Irene Campo, Maida Bada, Tommaso Silvestri. 2019. "Imaging" Mini-invasive Surgery. 3: 25. http://dx.doi.org/10.20517/2574-1225.2018.012
ACS Style
Cicero, C.; Casarin A.; Currò F.; Campo I.; Bada M.; Silvestri T. Imaging. Mini-invasive. Surg. 2019, 3, 25. http://dx.doi.org/10.20517/2574-1225.2018.012
About This Article
Copyright
Data & Comments
Data

 Cite This Article 4 clicks
Cite This Article 4 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.