Percutaneous mitral valve repair in acute mitral regurgitation: case report and review of the literature
Abstract
Acute mitral regurgitation is a heterogeneous and life-threatening pathology, with severe hemodynamic consequences and extremely adverse outcomes. Traditionally, the definitive treatment is prompt surgical intervention after hemodynamic stabilization. Nowadays, however, percutaneous repair of mitral valve with MitraClip device has emerged as a safe and effective therapeutic option. Evidences in this field are still scarce. Hereby, we report the case of an 82-year-old woman with lateral ST-elevation myocardial infarction determining severe acute mitral regurgitation (MR) with an asymmetric leaflet tethering mechanism. Due to prohibitive operative risk and unstable hemodynamic status, the patient underwent a successful urgent MitraClip procedure with optimal reduction of MR and immediate hemodynamic improvement. Moreover, we provide a review of the available literature regarding the echocardiographic assessment of acute MR, results of published cases and possible management of this complex pathology.
Keywords
Introduction
Percutaneous repair of mitral regurgitation (MR) with the MitraClip device (Abbott Vascular, Abbott Park, Illinois, USA) is an established therapeutic option for patients with prohibitive surgical risk and anatomically suitable mitral valve (MV)[1]. Implanted in over 100,000 patients worldwide, MitraClip procedure is safe and boasts a highly favourable risk-benefit ratio. While the impact of percutaneous MV repair on outcomes in chronic severe symptomatic MR has been evaluated for years in detail, data regarding the use of percutaneous edge-to-edge procedure in patients with severe acute MR are scarce and limited to case reports or small-size registries. Acute MR is a complex and heterogeneous pathology, with severe hemodynamic consequences and extremely adverse outcomes[2]. Traditionally, in most cases, after hemodynamic stabilization, the definitive treatment is surgical intervention. Nowadays, the MitraClip device is proving to be a valuable therapeutic option in high-risk patients.
Hereby, we present a case of acute severe ischemic mitral regurgitation successfully treated with MitraClip procedure.
Case description
We report the case of an 82-year-old female patient, who presented to emergency department for chest pain lasting for 72 h. The EKG revealed a latecomer lateral ST-elevation myocardial infarction, with ST-depression in V1-V4, ST-elevation and q waves in V7-V9. She had a history of arterial hypertension, rheumatoid arthritis, thalassemia minor, and radiotherapy-treated tongue cancer.
A bedside echocardiogram showed a left ventricular ejection fraction (LVEF) of 40% due to akinesia of posterior and lateral walls, normal left ventricular and atrial dimensions, mild MR, normal right ventricular function and size. Urgent coronary angiography was performed and showed a flow-limiting stenosis in the proximal tract of a dominant circumflex coronary artery. The coronary lesion was treated with balloon angioplasty and implantation of two drug-eluting stents. A severe no-reflow followed and prompted the use of intraortic balloon pump (IABP) for hemodynamic stabilization and the intracoronary injection of nitroprusside and adrenaline. The patient was transferred to Coronary Care Unit and remained hemodynamically stable for the subsequent 24 h.
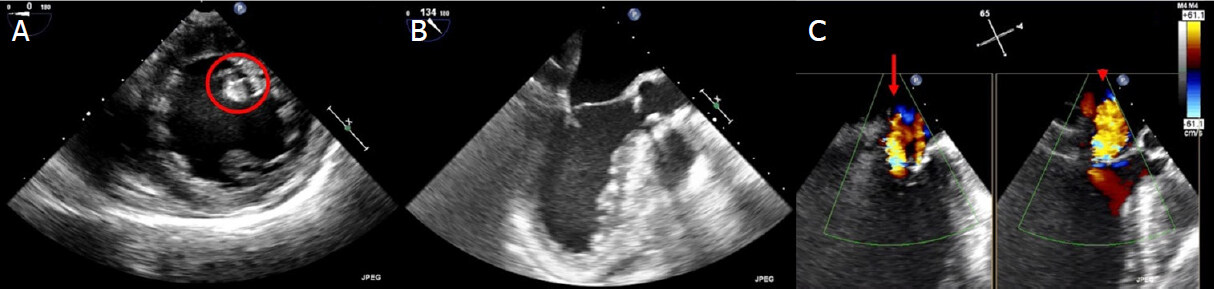
Then, a sudden hemodynamic collapse occurred, with pulmonary congestion and hypotension requiring intubation and high-dose vasopressors. Trans-thoracic and trans-esophageal echocardiogram (TEE) showed acute severe MR with eccentric jet directed towards the posterior wall of left atrium, due to extreme tethering of the posterior leaflet with partial posteromedial papillary muscle rupture and pseudoprolapse of the anterior leaflet [Figure 1]. The patient was deemed inoperable due to prohibitive surgical risk (age, subacute myocardial infarction with no-reflow injury, upper thorax radiotherapy, dual antiplatelet therapy, hemodynamic instability; STS score - risk of mortality: 66.6%; Euroscore II: 43.52%) and despite the highly challenging morphology of valvular disease, a salvage MitraClip procedure was the only feasible path. The mechanism of MR was complex: a Carpentier type IIIC (asymmetric systolic restriction) with a main jet located at A3-P3 extended to the medial section of A2-P2, plus a partial posteromedial papillary muscle rupture implicating an additional risk of mechanical complications, a coaptation gap > 10 mm, a posterior leaflet of 9 mm, but without calcifications at the grasping zone and with a suitable MV area (> 4 cm²)[3].
Figure 1. Baseline trans-esophageal echocardiogram showing partial postero-medial papillary muscle rupture (A, circle), extreme tethering of posterior leaflet with pseudoprolapse of anterior leaflet (B) and wide eccentric jet of severe mitral regurgitation mainly originating from A3-P3 (C, arrow) and extended to the medial section of A2-P2 (C, arrowhead)
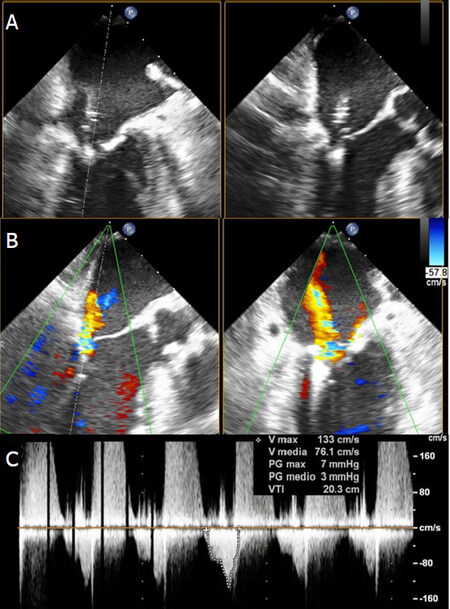
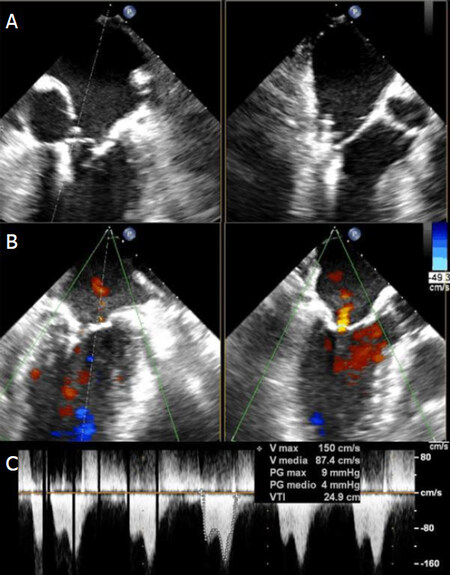
The patient underwent an urgent percutaneous edge-to-edge procedure under general anaesthesia, with IABP and vasopressor support, and using fluoroscopic and TEE guidance. An XTR Clip was first implanted in A3-P3 position with residual moderate MR and mean gradients of 3 mmHg [Figure 2], then an NTR Clip was used in A2-P2 position with a resulting minimal MR and mean gradients of 4 mmHg [Figure 3].
Figure 2. Intraprocedural trans-esophageal echocardiogram of XTR clip implantation: A3-P3 grasping (A), residual moderate mitral regurgitation located laterally to the clip (B) and transmitral gradients (C)
Figure 3. Intraprocedural trans-esophageal echocardiogram of NTR clip implantation: A2-P2 grasping (A), residual minimal mitral regurgitation (B) and transmitral gradients (C)
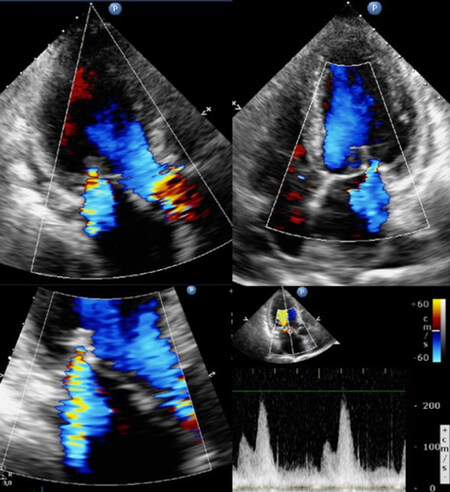
The patient’s hemodynamics progressively improved, and she was successfully weaned off mechanical ventilation and pharmacological support. Her post-operative recovery was uncomplicated and the patient was discharged on the tenth post-procedural day with residual mild MR and mean gradients of 5 mmHg [Figure 4].
Discussion
Acute MR is a medical and surgical emergency. Indeed, differently from chronic valvular diseases, acute MR occurs suddenly in normal sized hearts, without time for adaptative left atrial and ventricular enlargement. This results in a rapid increase of left atrial pressure with consequent pulmonary congestion and, despite initial hyperdynamic ventricular contraction, a risk of progressive reduction of cardiac output with hypotension and peripheral hypoperfusion[4]. Thus, patients with acute MR usually present with severe dyspnea, and slip towards cardiogenic shock.
Timely diagnosis may be insidious, due to nonspecific clinical pattern and equalization of left ventricular and atrial pressures leading to a soft or absent murmur[2]. Even pulmonary edema can be atypical with unilateral involvement if the regurgitant jet is eccentrically directed into either the right or the left pulmonary veins[2]. Echocardiography is key to diagnosis and proper management of the different causes of this disease[5].
Traditional management involves medical stabilization and surgical intervention, with a timing strictly related to the specific etiology of valve dysfunction[6]. MitraClip device has emerged as a new therapeutic alternative which is promising and potentially life-saving.
In the following sections, the main aspects of acute MR will be analysed with a focus on the amenability and use of percutaneous edge-to-edge repair technique in this condition.
Etiology
Identifying the precise mechanism and cause of acute MV disease is fundamental to tailor the most appropriate therapeutic strategy for each patient. Acute MR counts few mechanisms and many possible causes, as detailed in Table 1. First of all, the distinction between structural damages and functional alterations is fundamental, because organic causes always require repair, whereas functional causes may improve after targeting the underlying myocardial infarction, ischemia, or systolic dysfunction[5].
Classification of acute mitral regurgitation mechanisms and causes
| Mechanism | Cause |
|---|---|
| Organic/structural damage | |
| Carpentier type I (normal leaflet motion): perforation | Infective endocarditis
Device-related |
| Carpentier type II (excessive leaflet motion): prolapse/flail (papillary muscle rupture, chordal rupture) | Infective endocarditis
Myocardial ischemia Myxomatous degeneration Fibroelastic deficiency Idiopathic chordal rupture Device-related |
| Functional alteration | |
| Carpentier type III (restricted leaflet motion): symmetric/asymmetric systolic restriction | Myocardial ischemia |
| Carpentier type IV: systolic anterior motion of the leaflets | Hypertrophic cardiomyopathy
Takotsubo cardiomyopathy |
One major organic cause is chordal rupture which may occur in an otherwise totally normal valve or in a MV affected by Barlow’s disease or fibroelastic deficiency.
Device-related MR is a rare yet possible complication of left ventricular mechanical support devices due to catheter impingement in the chordal apparatus or leaflet tissue[7]. Iatrogenic MR is also reported after percutaneous mitral valvotomy for rheumatic mitral stenosis, albeit unlikely if patients are adequately selected.
Infective endocarditis can cause leaflet perforation and tears, papillary muscle and chordal rupture or may reduce systolic coaptation due to masses or abscesses interfering with leaflets’ apposition.
Extremely rare etiologies include chest traumas, systemic inflammatory diseases or acute rheumatic fever which remains a serious concern in endemic areas[8,9].
Ischemic papillary muscle rupture is another major cause of acute massive MR, with more frequent involvement of the posterior papillary muscle during inferior myocardial infarctions.
Acute myocardial ischemia or infarction may cause acute functional MR with systolic symmetrical or asymmetrical leaflet tethering, due to global or regional ventricular systolic dysfunction.
Another functional cause of MR is systolic anterior motion (SAM) of mitral leaflets in hypertrophic obstructive cardiomyopathy or Takotsubo cardiomyopathy[2].
Echocardiography
Diagnosis of acute MR
Echocardiography is essential for diagnosis. As opposed to chronic MR, left atrial and ventricular sizes are usually normal in acute MR, except for preexisting conditions influencing chambers’ dimension, compliance and hemodynamic tolerance. For instance, patients with a history of chronic MR and preserved ventricular systolic function have enlarged cardiac volumes and tolerate the further volumetric increase better than patients with normal sized hearts or with preexisting reduced LVEF. Color Doppler may underestimate the severity of MR, owing either to rapid equalization of left atrial and ventricular pressures or to an eccentric direction of the regurgitant jet with “Coanda” effect. Consequently, the use of color Doppler-based quantitative measures such as regurgitant volume and effective regurgitant orifice area may be misleading and even challenging, due to severe acute congestive heart failure with tachycardia. Vena contracta width and continuous wave Doppler signal represent reliable semiquantitative tools to quickly evaluate the significance of MR. A triangular and dense continuous wave Doppler curve supports the diagnosis of acute MR. It mirrors the rapid decline in late systolic velocity as a consequence of the abrupt increase in left atrial pressure. Systolic pulmonary venous flow reversal in one or both pulmonary veins can be found but tachycardia or atrial fibrillation can mask these findings. Any measure or value should be interpreted in the clinical context, as patients with acute heart failure and acute MR may appear to have only moderate MR when assessed by semi-quantitative and quantitative methods. Indeed, an acute significant MR should be suspected in patients with a clinical pattern of acute heart failure, with evidence of hyperdynamic LV without systolic or diastolic dysfunction, and with anatomic imaging of MV lesions[10,11].
Trans-thoracic echocardiography is the first-line examination in the assessment of acute dyspnea, feasible at bedside and sufficient to raise the clinical suspicion, but often inconclusive regarding the identification of the mechanism of MR, the evaluation of the MV anatomy and preoperative planning, which all require TEE. As such, three-dimensional (3D) echocardiography should always be adopted, as it provides anatomical details not detectable with two-dimensional (2D) imaging, enabling a dynamic and comprehensive assessment of MV tissue, and seizes dataset for off-line multiplanar reconstructions[12].
Mechanism and cause of MR
The first step of echocardiographic evaluation of MR mechanism is the distinction between organic/structural damage and functional alteration of MV [Table 1]. Close assessment of leaflet motion, anatomic lesions and finally the Color Doppler-based evaluation of convergence area and regurgitant jets are required.
A structural lesion with normal leaflet motion is generally due to a leaflet perforation. In this case, TEE should evaluate the position, shape and dimensions of the perforation, detect any sign suggestive of endocarditis, such as masses, vegetations or abscesses and explore mitral-aortic junction, left ventricular outflow tract and the position of other intracardiac devices. Indeed, an ambitious combined percutaneous procedure of MitraClip plus occluder would be contraindicated in the presence of active endocarditis, and an accurate preoperative planning should take into account the risk of iatrogenic obstruction of ventricular outflow or interference with proximally-located prostheses[13].
Leaflet flails and prolapse are categorised as Carpentier type II mechanism and occur through sudden rupture of chordae tendineae or papillary muscles due to many possible causes[11]. Myxoid degeneration or Barlow’s disease is a major cause of chordal rupture and remains extremely challenging for percutaneous repair due to altered anatomy, including extensive leaflet thickening, multi-segmental prolapse, elongated or fused chordae tendineae, diffuse calcifications and annular dilatation[14]. Beyond procedural challenges, the main issue is the balance between a relevant residual MR due to the highly mobile and redundant leaflets and a resultant iatrogenic mitral stenosis owing to extensive grasping with multiple clips. However, the introduction of MitraClip XTR device, with a wider reach and longer clip arms than NTR, has broadened the “graspable” MV anatomies, including Barlow’s disease, as documented by a few case series[15-17]. Nonetheless, myxoid degeneration appears early in life and patients are usually referred for surgery due to young age and low risk, on the contrary fibroelastic deficiency affects elderly people with significantly different operative risk. In fibroelastic deficiency MV is characterized by impaired production of connective tissue and shows thin leaflets, prolapse of single segments, and rupture of thin chordae with limited flail width[14]. MitraClip has been shown to be feasible and safe in this type of MV anatomy, even in octogenarians, but care should be taken in cases of fragile leaflet tissue due to the risk of grasping-related leaflet tears or lacerations[18].
Papillary muscle rupture is a severe, albeit rare, mechanical complication of acute myocardial infarction. This anatomic lesion is challenging given the large flail width and flail gap with frequent commissural localization requiring an extensive grasping with a concomitant high risk of chordal entanglement[19]. As a papillary muscle head may mimic an endocarditic mass, clinical context should guide the differential diagnosis[20]. Moreover, infective endocarditis itself may be causative of chordal rupture and papillary muscle laceration, in the presence of typical echocardiographic criteria such as vegetations and abscesses[21].
Among functional alterations of MV, the main cause of acute MR is myocardial ischemia. Indeed, in the very acute phase of myocardial infarction, even modest valve tenting due to regional and/or global left ventricular dysfunction may result in hemodynamically-significant MR[22]. Echocardiography should be performed to assess the presence of wall motion abnormalities and myocardial scarring, and the “symmetry” of mitral leaflets with respect to their point of coaptation. In cases of asymmetric tenting, it is generally the posterior leaflet that tethers while the anterior leaflet shows a ‘‘pseudoprolapse’’ motion. The MR jet is eccentric and oriented against the posterior wall of left atrium. In cases of symmetric tethering, both leaflets are tented but the coaptation point is displaced apically at the leaflets’ tips, and the jet is typically central[23].
An infrequent cause of acute MR is SAM of mitral leaflet, which represents a life-threatening condition and may result also in critical left ventricular outflow tract obstruction. Hypertrophic obstructive cardiomyopathy is the main pathology associated with SAM and is characterized by abnormalities of MV and subvalvular apparatus, such as malpositioned papillary muscles, elongated chordae and thickened leaflets[24]. These anatomic features may impact on transmitral gradients and residual MV area after MitraClip procedure[25]. SAM with left ventricular outflow obstruction may occur in several other conditions such as Takotsubo cardiomyopathy, hypertensive hypertrophic cardiopathy, hypovolemia, severe bleeding, sepsis, vasodilatation, sympathetic activation, pericardial tamponade, after aortic valve replacement, and after surgical mitral valve repair[25]. In these acute conditions a transcatheter edge-to-edge technique certainly sounds appealing to target both MR and hypotension.
Finally, rheumatic heart disease is commonly regarded as a contraindication to MitraClip procedures, owing to high risk of mitral stenosis. However, a recently published case report has shown the feasibility of percutaneous MV repair in a rheumatic MV with baseline mean gradients inferior to 4 mmHg[26]. Accurate measurement of MV area through 3D-based multiplanar reconstruction, evaluation of trans-mitral mean gradients and exclusion of calcifications at the grasping area are essential to decision-making and preoperative planning.
As outlined above, absolute anatomic limitations are very few. Hahn[27] listed the echocardiographic features associated with ideal and challenging anatomies and highlighted a few relative contraindications. The absolute contraindications include severe and extended calcifications of the grasping zone, short leaflet length (< 7 mm) and small baseline MV area (< 3.5 cm²)[15].
Real-world evidences of MitraClip procedure in acute MR
Real-world experience on percutaneous edge-to-edge repair of acute MR is uniquely derived from case reports and small-size registries, listed and synthetized in Table 2[19,28-43].
Case reports and registries of MitraClip in patients with acute MR
| Title | Ref. | Cause and mechanism of acute MR | Patients | Hemodynamic setting | Procedure and acute result | Early outcomes | Discharge | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Case reports | ||||||||
| Percutaneous mitral valve repair using the MitraClip in acute cardiogenic shock | Zuern et al.[29] | Acute cardiogenic shock and MOF in ischemic cardiomyopathy (2 previous anterior wall myocardial infarctions) | 51-year-old male
LVEF = 15% LVESD = 58 mm LVEDD = 67 mm BNP = 2786 ng/L | Cardiogenic shock and MOF
IABP and inotropes LAP = 36 mmHg PAP = 75/44 mmHg CO = 3.0 L/min | 1 clip (A2-P2)
MR grade = 1-2+ PCWP = 29 mmHg PAP = 66/37 mmHg CO = 4.3 L/min | Device success | Alive (postop day 7)
LVEF = 15% LVESD = 59 mm LVEDD = 68 mm sPAP = 32 mmHg BNP = 1,210 ng/L | 3-month
NYHA II MR grade = 1-2+ LVEF = 20% LVESD = 54 mm LVEDD = 63 mm sPAP = 34 mmHg BNP = 681 ng/L |
| Successful Percutaneous Mitral Valve Repair with the MitraClip System of Acute Mitral Regurgitation due to Papillary Muscle Rupture as Complication of Acute Myocardial Infarction | Bilge et al.[30] | Posterolateral STEMI, successful primary PCI of proximal circumflex artery with loss of marginal branch
Complete rupture of the anterolateral papillary muscle with A1-P1 flail and lateral jet | 60-year-old female
IABP LVEF = 45% | Pulmonary edema and cardiogenic shock
sPAP = 65 mmHg | 7 days after admission
1 clip (A1-P1) MR grade = 0 | Minor hemorrhagic stroke 2 days after MitraClip | Alive (postop day 9) | 30-day
MR grade = 1+ |
| MitraClip for Papillary Muscle Rupture in Patient With Cardiogenic Shock | Wolff et al.[31] | Latecomer lateral STEMI, with occlusion of large first obtuse marginal artery
Complete rupture of anterolateral papillary muscle with flail of A2 | 68-year-old male
LVEF = 25% Mild to moderate right ventricular dysfunction STS score = 64% EuroSCORE II = 75% | Cardiogenic shock and ventricular arrhythmias
IABP and intropes Mean LAP = 37 mmHg v-waves = 55 mmHg | 2 clips (A2-P2)
MR grade = 2+ v-wave = 30 mmHg | Device success | MR grade = 1-2+
LVEF = 30% LVEDD = 51 mm LVESD = 46 mm RV function = normal | 3-month
NYHA II MR grade = 1-2+ LVEF = 38% LVEDD = 62 mm LVESD = 50 mm 6-month NYHA II |
| MitraClip Implantation After Acute Ischemic Papillary Muscle Rupture in a Patient With Prolonged Cardiogenic Shock | Bahlmann et al.[32] | Lateral NSTEMI
Complete rupture of the posterior papillary muscle | 77-year-old male
Log Euroscore = 78% | Cardiogenic shock and pulmonary edema
IABP and inotropes Mean LAP = 21 mmHg Mean PAP = 24 mmHg Stroke volume index = 21 mL/m2 CO = 4.6 L/min CI = 2.2 L/min/m2 | 3 clips
MR grade = 0 Mean LAP = 22 mmHg Mean PAP = 26 mmHg Stroke volume index = 38 mL/m2 CO = 6.8 L/min CI = 3.2 L/min/m2 | Device success | Alive (postop day 16) | - |
| Percutaneous Mitral Valve Repair With Mitraclip System in a Patient With Acute Mitral Regurgitation After Myocardial Infarction | Rodríguez-Santamarta et al.[33] | Inferolateral STEMI, successful primary PCI of proximal circumflex artery
Ischemic asymmetric posterior leaflet tethering A2-P2 and A3-P3 | 76-year-old male
STS score = 6.7% Log Euroscore = 29.1% | Pulmonary edema | 2 clips (A2-P2; lateral to the first one)
MR grade = 1+ MV MG < 5 mmHg | MR grade (4th day) = 1+ | Alive
NYHA I | NYHA I |
| Effective Percutaneous “Edge-to-Edge” Mitral Valve Repair With MitraClip in a Patient With Acute Post-MI Regurgitation Not Related to Papillary Muscle Rupture | Tarsia et al.[34] | Inferior STEMI, successful primary PCI of right coronary artery and marginal branch, complete revascularization of left anterior descending artery after 48 hours
Ischemic symmetric leaflet tethering with central jet (A2-P2) | 65-year-old female
Log Euroscore = 42% | Cardiogenic shock and pulmonary edema
IABP and inotropes | 1 clip (A2-P2)
MR grade = 0 | Device success
No major complications | Alive (postop day 7) | 6-month
NYHA I MR grade = 0 |
| Acute Mitral Regurgitation Secondary to Papillary Muscle Tear Is Transcatheter Edge-to-Edge Mitral Valve Repair a New Paradigm? | Valle et al.[35] | Inferior STEMI, successful primary PCI of saphenous vein graft to right coronary artery
Partial tear of the posteromedial papillary muscle with flail of A2-A3 | 84-year-old male
LVEF = mildly reduced | Cardiogenic shock and MOF
Mean LAP = 29 mmHg v-wave = 59 mmHg | 3 clips in a “zipper” approach
MR grade = 1+ MV MG = 5 mmHg Mean LAP = 14 mmHg v-wave = 20 mmHg. | - | Alive | 6-week
NYHA II MR grade = 1-2+ |
| Use of MitraClip for Postmyocardial Infarction Mitral Regurgitation Secondary to Papillary Muscle Dysfunction | Yasin et al.[36] | Inferior NSTEMI
Partial rupture of posteromedial papillary muscle with flail of posterior leaflet | 68-year-old male | Cardiogenic shock and pulmonary edema
v-a ECMO | 5 day after admission
2 clips (A2-P2, P1-P2) MR grade = 1+ | MR grade (3th day) = 1+
Device success | Alive | 30-day
MR grade = 1+ |
| Edge-to-edge mitral valve repair for acute mitral valve regurgitation due to papillary muscle rupture: a case report | Papadopoulos et al.[19] | Anterior STEMI, successful primary PCI of intermediate artery
Partial rupture of the anterolateral papillary muscle with flail of A1-A2 and P1 | 85-year-old female
Log Euroscore = 43% STS score = 13% LVEF = 40% | Cardiogenic shock and pulmonary edema
IABP and inotropes | 2 clips (A2-P2, A1-P1) with a “zipping” of the lateral commissure
MR grade = 1-2+ MV area = 2.1 cm2 MV MG = 6 mmHg | Device success | Alive (postop day 7) | 20-month
NYHA II MR grade = 2+ MV MG = 6 mmHg |
| One-stop-shop totally percutaneous approach for severe aortic and mitral regurgitation in cardiogenic shock | Pagnotta et al.[37] | Latecomer anterior STEMI, successful primary PCI of proximal right coronary artery and left circumflex artery (proximal left anterior descending artery occluded)
Retraction and calcification of posterior mitral leaflet and severe aortic regurgitation in a mediastinal radiotherapy-related valvular heart disease | 57-year-old male
LVEF = 30% Log Euroscore = 17.7% | Cardiogenic shock
IABP and inotropes | 1 XTR clip
MR grade = 1+ MV MG = 5 mmHg | - | Alive | 30-day
MR grade = 1+ MV MG = 5 mmHg LVEF = 35% |
| Successful MitraClip XTR for Torrential Mitral Regurgitation Secondary to Papillary Muscle Rupture as a Complication of Acute Myocardial Infarction | Villablanca et al.[38] | Lateral NSTEMI, successful primary PCI of proximal and mid-circumflex artery
Complete rupture of posteromedial papillary muscle with flail of P2-P3 | 70-year-old male
LVEF = 60% STS score = 14.3% | Cardiogenic shock and pulmonary edema
Impella CP, then exchanged with IABP plus inotropes Mean LAP = 22 mmHg v-wave = 60 mmHg CO = 3.7 L/min CI = 1.8 L/min/m2 | 1 XTR clip (A2-P2)
MR grade = 1+ MV MG = 1 mmHg Mean LAP = 10 mmHg v-wave = 12 mmHg, CO = 4.9 L/min CI = 2.8 L/min/m2 | - | Alive (postop day 3) | 6-month
NYHA I MR grade = 1+ |
| Transcatheter Mitral Valve Edge-to-Edge Repair with the New MitraClip XTR System for Acute Mitral Regurgitation Caused by Papillary Muscle Rupture | Komatsu et al.[28] | Inferior STEMI, successful primary PCI of culprit single-vessel disease
Posteromedial papillary muscle rupture with anteriorly directed eccentric jet Coaptation gap = 1 cm MV area = 6.2 cm2 MV MG = 3 mmHg | 55-year-old male
LVEF = 55% | Pulmonary edema, cardiogenic shock and acute kidney injury
IABP and vasopressors V wave = 50 mmHg | 2 clip XTR (A2-P2)
MR grade = 1-2+ MV MG = 3 mmHg MV area = 2.94 cm2 V wave = 17 mmHg | - | Alive | 3-month
MR grade = 2+ (eccentric) No HF symptoms |
| Case series or registries | ||||||||
| Percutaneous Mitral Valve Repair for Acute Mitral Regurgitation After an Acute Myocardial Infarction | Estévez-Loureiro et al.[39] | AMI without papillary muscle rupture:
Days between MI and clip:
| N of patients = 5
Age = 51 – 76 years Median Euroscore = 29.1% 5/187 MitraClip procedures (2.7%) Period: 10/2010-01/2015 | Cardiogenic shock = 3
NYHA IV = 2 IABP or inotropes = 4 Median sPAP = 62 mmHg | N of clips:
MV area > 1.5 cm2 = 5 (100%) MV MG < 5 mmHg = 5 (100%) MR grade ≤ 2+ = 5 (100%) | Device success = 5/5
Median sPAP = 38 mmHg No major complications | Deaths = 1 (20%) (due to MOF 1 week after MitraClip) | Median follow-up = 317 days
NYHA class
MR grade
|
| Percutaneous edge-to-edge mitral valve repair for the treatment of acute mitral regurgitation complicating myocardial infarction: A single centre experience | Adamo et al.[40] | AMI without papillary muscle rupture:
| N of patients = 5
Age = 73 ± 6 years Males = 3 Median Euroscore = 27.1 ± 13% Median STS score = 10.2 ± 6% 5/79 MitraClip procedures (6.3%) Period: 10/2010-10/2015 | Cardiogenic shock = 4
Pulmonary edema = 1 IABP and inotropes = 4 Inotropes = 1 | 53 ± 33 days from admission
N of clips:
| Device success = 5/5 | Deaths = 0 | 1 death due to non-cardiovascular causes 57 days after MitraClip
1 left-ventricular assist device implantation 60 days after MitraClip |
| Percutaneous edge-to-edge mitral valve repair may rescue select patients in cardiogenic shock: findings from a single center case series | Flint et al.[41] | Cardiogenic shock:
| N of patients = 12
Age = 71.7 ± 12.8 years Males = 9 LVEF = 46 ± 12% STS score (MV repair) = 33.4 ± 22.3% STS score (MV replacement) = 23.9 ± 18.2% 12/135 MitraClip procedures (9%) Period: 11/2013-10/2018 | Cardiogenic shock
-IABP + inotropes = 3 -IABP + nitroprusside = 1 -inotropes + nitroprusside = 1 -inotropes = 6 -nitroprusside = 1 -ECMO = 1 Mean LAP = 27 ± 9 Mean PAP = 38 ± 11 Mean sPAP = 57 ± 17 Mean RAP = 13 ± 5 CI = 2.2 ± 0.5 CO = 4.3 ± 1.2 | N of clips = 2.3 ± 0.7
MR grade
MV MG = 5.0 ± 2.7 mmHg LVEDD = 5.4 ± 0.8 cm LVEF = 37 ± 15% | - | Death (6th day) = 1
Resolution of shock = 10 Inotrope-dependent = 1 | Deaths = 4 (26-282 days) |
| Salvage MitraClip in severe secondary mitral regurgitation complicating acute myocardial infarction: data from a multicentre international study | Haberman et al.[42] | AMI within 90 days:
No evidence of structural valvular damage | N of patients = 20
Age = 68 ± 10 years Males = 6 (30%) LVEF = 35.9 ± 12.5% LVEF < 30% = 7 Period: 01/2011-09/2018 | Cardiogenic shock = 8
sPAP = 60 ± 12 mmHg v-wave = 31 ± 25 mmHg | Days after MI = 32 (7-90)
Clips = 1 – 3 MR grade 1+ = 12 MR grade 2+ = 7 PAP = 40 ± 13 mmHg v-wave = 17 ± 5 mmHg | Device success = 19/20
In one patient, a posterior leaflet tear occurred after the second clip implantation and urgent MV replacement surgery was performed but the patient died. | Death = 1 | Median follow-up = 15 months
Death = 1 (3 weeks after discharge) |
| Transcatheter mitral valve repair in patients with acute
myocardial infarction: insights from the European Registry of MitraClip in Acute Mitral Regurgitation following an acute myocardial infarction (EREMMI) | Estévez-Loureiro et al.[43] | STEMI
Primary PCI = 30 (68.2%) MR grade
MR jet location:
| N of patients = 44
Age = 70.0 ± 10.8 years Males = 63.6% Euroscore II = 15.1% (6.2-23.2) LVEF = 35% (26-44) LVEDD = 55.5 (48.2-59.5) mm 44/883 MitraClip procedures (5%) Period: 01/2016-12/2018 | Mechanical support
Inotropes = 24 (54.5%) sPAP = 52.5 (25-77.5) mmHg TAPSE = 16.5 (16-20.3) mm | Median n of clips = 2 (1-2)
Median MV MG = 3 mmHg (2-4) Median time from MI = 18 days (13-36.8) | Device success = 86.6%
Median length of stay after procedure = 16 (8-27) days | - | 30-day
Death = 4 (9.1%) Cardiac surgery = 1 (2.3%) 6-month Death = 8 (18.2%) HF rehosp = 8 (18.2%) Cardiac surgery = 3 (6.8%) MR grade
NYHA
|
The vast majority of cases occurred as complications of acute myocardial infarction, due to either ischemic leaflet tethering or papillary muscle ruptures (more often the posteromedial one). A primary PCI was always performed, except for late presentations due to the likely risk of reperfusion injury of already necrotic walls. The hemodynamic status was generally critical with evidence of cardiogenic shock and pulmonary edema requiring intubation, inotropes and mechanical support, mainly IABP and only in few cases veno-arterial extracorporeal membrane oxygenation. The Heart Team’s decision to proceed with MitraClip was primarily guided by the high surgical risk due to hemodynamic instability, acute/subacute ischemia, dual antiplatelet therapy, advanced age or comorbidities in a few cases, and the favourable risk-benefit balance of percutaneous edge-to-edge approach. Hemodynamic stabilization and likeliness of MR improvement with revascularization or medical therapy were the main determinants of the timing of procedure. One to three clips were deployed with an almost complete procedural success, due to significant reduction of MR, huge reduction of left atrial pressures and increase of cardiac output. Haberman et al.[42] reported one case of posterior leaflet tear during a second clip implantation, followed by urgent MV surgery and lastly by patient death. Early outcomes were promising, with high survival rates, sustained reduction of MR grade and improved functional class. These results are even more reassuring if compared with surgical ones; in a multicentre surgical registry of 279 patients treated with emergency surgery for acute severe MR, due to myocardial infarction, acute endocarditis or degenerative MV disease, the 30-day mortality was 22.5% with worse survival rates in case of acute myocardial infarction, endocarditis, shock, coronary artery disease and systolic dysfunction[44]. Despite the evidences seem extremely optimistic regarding outcomes of MitraClip in almost every acute setting, a publication bias has to be recognized, and the interventionalists’ and imagers’ experience should be considered during Heart Team decision-making. Certainly, MitraClip shows several advantages over surgery. Firstly, MitraClip is safe and does not preclude a delayed surgical procedure in case of failure, thus a “bridge” procedure may be always attempted without significant additional risks. Secondly, percutaneous procedures permit to avoid the cardiopulmonary bypass and the associated systemic inflammatory storm and myocardial oxidative stress. Furthermore, transcatheter procedures do not cause abnormal motion of the right ventricle or interventricular septum, which may impact on long-term LV performance[43].
Decision-making and management
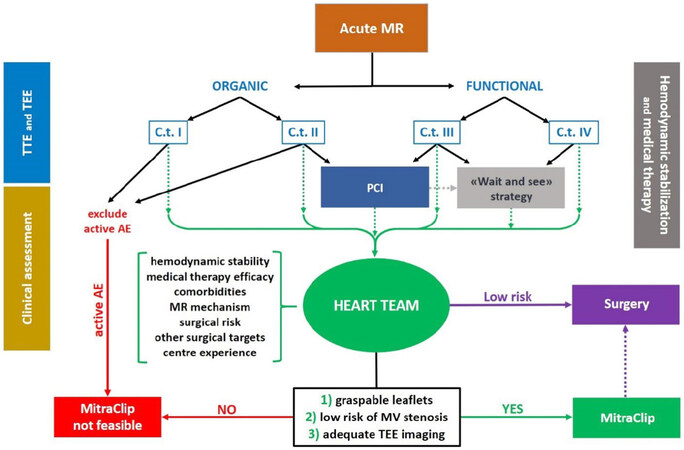
We propose a flow-chart that may be helpful for acute MR decision-making and management [Figure 5].
Figure 5. Proposed flow-chart for acute MR decision-making and management. “Carpentier type” refers to Carpentier classification of MR mechanisms, as exposed in Table 1. AE: acute endocarditis; C.t.: Carpentier type; MR: mitral regurgitation; MV: mitral valve; PCI: percutaneous coronary intervention; TEE: trans-esophageal echocardiography; TTE: trans-thoracic echocardiography
Once acute MR is diagnosed, the initial goal is hemodynamic stabilization through inotropes/vasopressors and temporary mechanical circulatory supports (IABP, Impella and extracorporeal membrane oxygenation) in cases of cardiogenic shock, intravenous diuretic therapy and non-invasive/invasive ventilation for massive pulmonary edema with acute respiratory distress. Hypertensive or normotensive patients benefit from afterload reduction with intravenous vasodilator therapy, which reduces MR, diminishing pulmonary congestion and increases forward cardiac output[6].
Then, an accurate trans-esophageal echocardiographic characterization of acute MR is needed to tailor the subsequent actions. The finding of a Carpentier type I lesion, as a leaflet perforation, should lead to exclude an active acute endocarditis, which represents a contraindication for MitraClip procedure and an indication for cardiac surgery[45]. Clinical context and laboratory tests are pivotal to distinguish active endocarditic processes from a treated state, for which percutaneous interventions are not contraindicated. Careful history taking is sufficient to exclude a device-related (transcatheter prosthetic aortic valves and intracardiac left ventricular assist devices) mechanism.
A Carpentier type II mechanism (leaflet prolapse/flail, rupture of chordae or papillary muscle) warrants a wide differential diagnosis between multiple potential causes. As already reported for Carpentier type I, active endocarditic processes should be excluded, as well. Myocardial ischemia or infarction may cause partial or complete rupture of a papillary muscle and could be targeted with medical therapy or percutaneous myocardial revascularization, before treating the MV lesion. A case-by-case judgement is fundamental and should consider the presence of ongoing myocardial ischemia, the timing of onset of myocardial infarction, the extension of coronary artery disease, the type of MV lesion (partial versus complete rupture), and the differential burden between ischemic and valvular diseases. Primary percutaneous coronary intervention for an ST-elevation myocardial infarction is always indicated, except for delayed infarctions without evidence of ongoing ischemia, as they would not yield significant benefits from revascularization and yet be complicated by reperfusion injury[46,47]. Early surgical intervention is crucial for complete papillary muscle rupture, although partial rupture may benefit from percutaneous revascularization or a brief period of stabilization[48]. When acute MR is caused by chordal rupture in MV affected by fibroelastic deficiency or Barlow’s disease, anatomic evaluation has a central role to ensure the feasibility of an eventual MitraClip procedure, as already explained in previous paragraphs.
The observation of a Carpentier type III mechanism is related to regional or global LV systolic dysfunction. Medical therapy and percutaneous coronary intervention can acutely reduce the degree of ischemic MR, and an earlier reperfusion time is associated with greater reduction in MR severity[49]. Thus, if hemodynamic conditions are stable after revascularization or medical therapy implementation, a “wait and see” strategy may be undertaken with close and constant monitoring.
A Carpentier type IV mechanism, namely a SAM of mitral leaflets, observed in hypertrophic and Takotsubo cardiomyopathies, represents an insidious cause of acute MR. Echocardiographic diagnosis is exceedingly important, as vasodilators, inotropes or IABP worsen the clinical and hemodynamic status. Beta-blockers, volume expansion, inotrope discontinuation, afterload augmentation with vasopressors and lastly mechanical circulatory supports (Impella and extracorporeal membrane oxygenation) are the weapons to turn to[50,51].
After the initial phases of echocardiographic diagnosis, hemodynamic stabilization, medical therapy implementation and eventually percutaneous coronary revascularization, it is time for Heart Team assessment. Interventionalists, cardiac surgeons, imagers, intensivists and heart failure specialists must meet to tailor the best therapeutic pathway, weighing all clinical and anatomical factors: age, comorbidities, hemodynamic status, response to medical therapy, MR mechanism, surgical risk, other surgical targets and single centre’s experience. In cases of low surgical risk, presence of an indication for concomitant cardiac surgery and organic MV disease, cardiac surgery is the first-choice treatment. Differently, in our opinion, MitraClip should be always attempted in a stepwise approach, as it is a safe procedure and does not preclude a delayed surgical intervention. Even in low-risk patients undergoing isolated MV surgery with a low probability of surgical repair, MitraClip may be attempted, above all in high-volume centers. Eligibility for percutaneous edge-to-edge procedure requires only three conditions: possibility to grasp and approximate the leaflets, low risk of MV stenosis, and good-quality TEE imaging[19].
Conclusion
Acute MR is a life-threatening condition, traditionally treated as a medical and surgical emergency. Percutaneous edge-to-edge repair of MV is a safe and effective therapeutic option, does not preclude delayed cardiac surgery and is potentially able to solve almost any type of MV disease, with very few contraindications. Echocardiographic identification of the precise valvular lesion and Heart Team evaluation are pivotal to tailor the best therapeutic pathway for each patient. Literature confirms optimal results of MitraClip in acute MR, but further studies are warranted to shed light on feasibility and limitations of this powerful procedure.
Declarations
Authors’ contributionsInvolved in clinical care: Sanz-Sánchez J, Chiarito M, Briani M, Fazzari F, Corrada E, Bragato RM, Pagnotta PA, Regazzoli D
Wrote the manuscript: Cannata F, Regazzoli D
Supervised and coordinated all aspects of the research: Stefanini GG, Reimers B
Contributed to critical revision of the manuscript and approved the final version of the manuscript: Cannata F, Sanz-Sánchez J, Chiarito M, Briani M, Fazzari F, Bertoldi LF, Ferrante G, Corrada E, Bragato RM, Stefanini GG, Pagnotta PA, Reimers B, Regazzoli D
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationA written informed consent for publication was obtained.
Copyright© The Author(s) 2020.
REFERENCES
1. Khan F, Wintel F, Ong G, Brugger N, Pilgrim T, et al. Percutaneous mitral edge-to-edge repair: state of the art and a glimpse to the future. Front Cardiovasc Med 2019;6:122.
3. Nyman C, Mackensen GB, Jelacic S, Little SH, Smith Tw, et al. Transcatheter mitral valve repair using the edge-to-edge clip. J Am Soc Echocardiogr 2018;31:434-53.
4. Gaasch WH, Meyer TE. Left ventricular response to mitral regurgitation: implications for management. Circulation 2008;118:2298-303.
7. Khalid N, Shlofmitz E, Case BC, Waksman R. Chordae tendineae rupture and iatrogenic severe mitral regurgitation related to impella. EuroIntervention 2020; doi: 10.4244/EIJ-D-19-00942.
8. Saric P, Ravaee BD, Patel TR, Hoit BD. Acute severe mitral regurgitation after blunt chest trauma. Echocardiography 2018;35:272-4.
9. Kaymaz C, Ozdemir N, Ozkan M. Differentiating clinical and echocardiographic characteristics of chordal rupture detected in patients with rheumatic mitral valve disease and floppy mitral valve: impact of the infective endocarditis on chordal rupture. Eur J Echocardiogr 2005;6:117-26.
10. Lancellotti P, Zamorano J, Badano L, Habib G. The EACVI Textbook of Echocardiography. 2nd ed. Oxford University Press; 2016.
11. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, et al. Recommendations for non-invasive evaluation of native valvular regurgitation: a report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J Am Soc Echocardiogr 2017;30:303-71.
12. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95.
13. Javed U, Smith TW, Rogers JH. Percutaneous repair of anterior mitral leaflet perforation. J Invasive Cardiol 2012;24:134-7.
14. Anyanwu AC, Adams DH. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg 2007;19:90-6.
15. Khalique OK, Hahn RT. Percutaneous mitral valve repair: multi-modality cardiac imaging for patient selection and intra-procedural guidance. Front Cardiovasc Med 2019;6:142.
16. Weinmann K, Markovic S, Rottbauer W, Keßler M. First experience with the MitraClip XTR device for extensive mitral valve prolapse (Barlow’s disease). EuroIntervention 2018;14:1276-7.
17. Lee CW, Sung SH, Huang WM, Tsai YL, Hsu CP, et al. Clipping Barlow’s mitral valve to rescue a patient with acute biventricular failure. AsiaIntervention 2019;5:64-7.
18. Geis N, Raake P, Mereles D, Chorianopoulos E, Szabo G, et al. Percutaneous repair of severe mitral valve regurgitation secondary to chordae rupture in octogenarians using MitraClip. J Interv Cardiol 2018;31:76-82.
19. Papadopoulos K, Chrissoheris M, Nikolaou I, Spargias K. Edge-to-edge mitral valve repair for acute mitral valve regurgitation due to papillary muscle rupture: a case report. Eur Heart J Case Rep 2019;3:1-4.
20. Shapiro MD, Leibowitz K, Hanon S, Berger M, Schweitzer P. Papillary muscle masquerading as a vegetation. Int J Cardiol 2006;113:106-7.
21. Evangelista A, Gonzalez-Alujas MT. Echocardiography in infective endocarditis. Heart 2004;90:614-7.
22. Nishino S, Watanabe N, Kimura T, Kuriyama N, Shibata Y. Acute versus chronic ischemic mitral regurgitation: an echocardiographic study of anatomy and physiology. Circ Cardiovasc Imaging 2018;11:e007028.
23. Silbiger JJ. Mechanistic insights into ischemic mitral regurgitation: echocardiographic and surgical implications. J Am Soc Echocardiogr 2011;24:707-19.
24. Sherrid MV, Balaram S, Kim B, Axel L, Swistel DG. The mitral valve in obstructive hypertrophic cardiomyopathy: a test in context. J Am Coll Cardiol 2016;67:1846-58.
25. Schäfer U, Frerker C, Thielsen T, Schewel D, Bader R, et al. Targeting systolic anterior motion and left ventricular outflow tract obstruction in hypertrophic obstructed cardiomyopathy with a MitraClip. EuroIntervention 2015;11:942-7.
26. Wong N, Tan PCM, Ding ZP, Yeo KK. Successful MitraClip for severe rheumatic mitral regurgitation: a case report. Eur Heart J Case Rep 2019;3:ytz155.
27. Hahn RT. Review of procedures and intraprocedural echocardiographic imaging. Circ Res 2016;119:341-56.
28. Komatsu I, Cohen EA, Cohen GN, Czarnecki A. Transcatheter mitral valve edge-to-edge repair with the new MitraClip XTR system for acute mitral regurgitation caused by papillary muscle rupture. Can J Cardiol 2019;35:1604.e5-7.
29. Zuern CS, Schreieck J, Weig HJ, Gawaz M, May AE. Percutaneous mitral valve repair using the MitraClip in acute cardiogenic shock. Clin Res Cardiol 2011;100:719-21.
30. Bilge M, Alemdar R, Yasar AS. Successful percutaneous mitral valve repair with the MitraClip system of acute mitral regurgitation due to papillary muscle rupture as complication of acute myocardial infarction. Catheter Cardiovasc Interv 2014;83:E137-40.
31. Wolff R, Cohen G, Peterson C, Wong S, Hockman E, et al. MitraClip for papillary muscle rupture in patient with cardiogenic shock. Can J Cardiol 2014;30:1461.e13-4.
32. Bahlmann E, Frerker C, Kreidel F, Wong S, Hockman E, et al. MitraClip implantation after acute ischemic papillary muscle rupture in a patient with prolonged cardiogenic shock. Ann Thorac Surg 2015;99:e41-2.
33. Rodríguez-Santamarta M, Estévez-Loureiro R, Gualis J, Alonso D, Pérez de Prado A, et al. Percutaneous mitral valve repair with MitraClip system in a patient with acute mitral regurgitation after myocardial infarction. Rev Esp Cardiol (Engl Ed) 2015;68:259-61.
34. Tarsia G, Smaldone C, Costantino MF. Effective percutaneous “edge-to-edge” mitral valve repair with mitraclip in a patient with acute post-MI regurgitation not related to papillary muscle rupture. Catheter Cardiovasc Interv 2016;88:1177-80.
35. Valle JA, Miyasaka RL, Carroll JD. Acute mitral regurgitation secondary to papillary muscle tear: is transcatheter edge-to-edge mitral valve repair a new paradigm? Circ Cardiovasc Interv 2017;10:e005050.
36. Yasin M, Nanjundappa A, Annie FH, Tager A, Farooq A, et al. Use of MitraClip for postmyocardial infarction mitral regurgitation secondary to papillary muscle dysfunction. Cureus 2018;10:e3065.
37. Pagnotta PA, Sanz-Sánchez J, Regazzoli D, Ferrante G. One-stop-shop totally percutaneous approach for severe aortic and mitral regurgitation in cardiogenic shock. Catheter Cardiovasc Interv 2020;95:411-3.
38. Villablanca PA, Wang DD, Lynch D, O’Neil W, Eng M. Successful MitraClip XTR for torrential mitral regurgitation secondary to papillary muscle rupture as a complication of acute myocardial infarction. Struct Heart Dis 2019;3:352-5.
39. Estévez-Loureiro R, Arzamendi D, Freixa X, Cardenal R, Carrasco-Chinchilla F, et al. Percutaneous mitral valve repair for acute mitral regurgitation after an acute myocardial infarction. J Am Coll Cardiol 2015;66:91-2.
40. Adamo M, Curello S, Chiari E, Fiorina C, Chizzola G, et al. Percutaneous edge-to-edge mitral valve repair for the treatment of acute mitral regurgitation complicating myocardial infarction: a single centre experience. Int J Cardiol 2017;234:53-7.
41. Flint K, Brieke A, Wiktor D, Carroll J. Percutaneous edge-to-edge mitral valve repair may rescue select patients in cardiogenic shock: findings from a single center case series. Catheter Cardiovasc Interv 2019;94:E82-7.
42. Haberman D, Taramasso M, Czarnecki A, Kerner A, Chrissoheri M, et al. Salvage MitraClip in severe secondary mitral regurgitation complicating acute myocardial infarction: data from a multicentre international study. Eur J Heart Fail 2019;21:1161-4.
43. Estévez-Loureiro R, Adamo M, Arzamendi D, Denti P, Freixa X, et al. Transcatheter mitral valve repair in patients with acute myocardial infarction: insights from the European Registry of MitraClip in Acute Mitral Regurgitation following an acute myocardial infarction (EREMMI). EuroIntervention 2020;15:1248-50.
44. Lorusso R, Gelsomino S, De Cicco G, Beghi C, Russo C, et al. Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg 2008;33:573-82.
45. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Eur Heart J 2015;36:3075-128.
46. Chua S, Hung J, Chung SY, Lin YC, Fu M, et al. Primary percutaneous coronary intervention lowers the incidence of ischemic mitral regurgitation in patients with acute ST-elevated myocardial infarction. Circ J 2010;74:2386-92.
47. MacHaalany J, Bertrand OF, O’Connor K, Abdelaal E, Voisine P, et al. Predictors and prognosis of early ischemic mitral regurgitation in the era of primary percutaneous coronary revascularisation. Cardiovasc Ultrasound 2014;12:14.
48. Chevalier P, Burri H, Fahrat F, Cucherat M, Jegaden O, et al. Perioperative outcome and long-term survival of surgery for acute post-infarction mitral regurgitation. Eur J Cardiothorac Surg 2004;26:330-5.
49. Nishino S, Watanabe N, Kimura T, Enriquez-Sarano M, Nakama T, et al. The course of ischemic mitral regurgitation in acute myocardial infarction after primary percutaneous coronary intervention: from emergency room to long-term follow-up. Circ Cardiovasc Imaging 2016;9:e004841.
50. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1955-71.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Cannata F, Sanz-Sánchez J, Chiarito M, Briani M, Fazzari F, Bertoldi LF, Ferrante G, Corrada E, Bragato RM, Stefanini GG, Pagnotta PA, Reimers B, Regazzoli D. Percutaneous mitral valve repair in acute mitral regurgitation: case report and review of the literature. Mini-invasive Surg 2020;4:53. http://dx.doi.org/10.20517/2574-1225.2020.41
AMA Style
Cannata F, Sanz-Sánchez J, Chiarito M, Briani M, Fazzari F, Bertoldi LF, Ferrante G, Corrada E, Bragato RM, Stefanini GG, Pagnotta PA, Reimers B, Regazzoli D. Percutaneous mitral valve repair in acute mitral regurgitation: case report and review of the literature. Mini-invasive Surgery. 2020; 4: 53. http://dx.doi.org/10.20517/2574-1225.2020.41
Chicago/Turabian Style
Cannata, Francesco, Jorge Sanz-Sánchez, Mauro Chiarito, Martina Briani, Fabio Fazzari, Letizia F. Bertoldi, Giuseppe Ferrante, Elena Corrada, Renato M. Bragato, Giulio G. Stefanini, Paolo A. Pagnotta, Bernhard Reimers, Damiano Regazzoli. 2020. "Percutaneous mitral valve repair in acute mitral regurgitation: case report and review of the literature" Mini-invasive Surgery. 4: 53. http://dx.doi.org/10.20517/2574-1225.2020.41
ACS Style
Cannata, F.; Sanz-Sánchez J.; Chiarito M.; Briani M.; Fazzari F.; Bertoldi LF.; Ferrante G.; Corrada E.; Bragato RM.; Stefanini GG.; Pagnotta PA.; Reimers B.; Regazzoli D. Percutaneous mitral valve repair in acute mitral regurgitation: case report and review of the literature. Mini-invasive. Surg. 2020, 4, 53. http://dx.doi.org/10.20517/2574-1225.2020.41
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 19 clicks
Cite This Article 19 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.