Robot-assisted spleen preserving distal pancreatectomy (RA-SPDP): a single center experience
Abstract
Aim: To define the outcome of robot-assisted spleen preserving distal pancreatectomy (RA-SPDP) in a high-volume center.
Methods: A retrospective analysis of a prospectively maintained database was performed to identify RA-SPDP performed at our Center between April 2008 to October 2017.
Results: During the study period, RA-SPDP was attempted in 54 patients. The spleen was preserved, always along with the splenic vessels (Kimura procedure), in 52 patients (96.3%). There were no conversions to open or laparoscopic surgery. Mean operative time was 260 min (231.3-360.0). Grade B post-operative pancreatic fistula (POPF) occurred in 19 patients (35.2%). There were no grade C POPF. Two patients required repeat surgery because of postoperative bleeding and splenic infarction, respectively. There were no post-operative deaths at 90 days. Excluding one patient with known diagnosis of metastasis from renal cell carcinoma, malignancy was eventually identified in 7 of 53 patients (13.2%).
Conclusion: In the hands of dedicated pancreatic surgeons, robotic assistance results in a high rate of spleen preservation with good clinical outcomes. Despite careful preoperative selection, several patients can be found to have a malignant tumor. Taken altogether these results suggest that patients requiring these procedures should be preferentially referred to specialized centers.
Keywords
Introduction
Based on current evidence, and recommendations, a minimally invasive approach should be offered to patients with benign or borderline tumors located in the body-tail of the pancreas[1]. Actually, in recent years, distal pancreatectomy gained so much popularity as to be considered the “standard of care” by some authors for resectable pancreatic tumors located in the distal part of the pancreas[2].
Little doubt exists that the spleen should be preserved, whenever oncologically indicated and anatomically possible, to reduce the rate of infective[3-5] and thromboembolic complications[5,6], and to improve blood supply to the proximal part of the stomach[7]. During distal pancreatectomy the spleen can be preserved along with the splenic vessels (Kimura technique)[8] or with en-bloc removal of the splenic vessels (Warshaw technique)[9].
The da Vinci Surgical System® (Intuitive Surgical Inc. Sunnyvale, CA, USA) is a telemanipulator that faithfully transmits the movements of surgeon’s hands to the miniaturized tips of intracorporeal instruments with seven degrees of freedom[10]. Thanks to this tremendous technological improvement, as well as to some other advances, the da Vinci robot was shown to improve surgical dexterity in minimally invasive procedures[11]. Based on this background the use of the da Vinci robot seems to be particularly rewarding when the spleen and the splenic vessels must be preserved during distal pancreatectomy. We herein present our series of robot-assisted spleen preserving distal pancreatectomy (RA-SPDP).
Methods
A retrospective analysis of a prospectively maintained database was performed to identify patients who were selected for RA-SPDP and received this procedure between April 2008 to July 2019 at a single Institution (Division of General and Transplant Surgery, University of Pisa). Data were collected and analyzed according to the Strengthening the Reporting of Observational studies in Epidemiology guidelines for observational studies[12].
Patient selection
Indications to distal pancreatectomy with spleen preservation was established by a multidisciplinary team, annually managing several hundreds of patients with pancreatic diseases. Distal pancreatectomy with spleen preservation was considered in patients with benign tumors causing symptoms or in patients with tumors of low malignant potential located in the body-tail of the pancreas. A minimally invasive approach was considered in each patient unless obviously impossible. A robotic approach was considered whenever the robot was timely available. Alternatively, patients received a laparoscopic procedure. Absolute contraindications to RA-SPDP were thrombosis of the splenic vein, tumors size exceeding 15 cm, concurrent splenic disease, and concerns on tumor type.
All patients underwent standard preoperative work-up and were assigned to one of the risk categories defined by the American Society of Anesthesia[13]. Pancreatic tumors were studied extensively using a combination of laboratory and imaging studies as required by the individual case until a final diagnosis was agreed upon by a group of experts, including surgeons, oncologists, and radiologists. Endoscopic ultrasound, with fine needle aspiration cytology or biopsy, was also employed as required.
Surgical technique
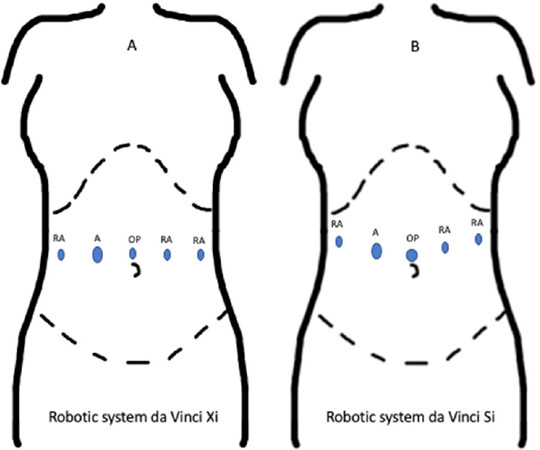
Patients were placed supine on an operating table equipped with a thermic blanket with the legs parted (French position). Intermittent pneumatic compression cuffs were placed around the legs and patients were secured to the operating table with wide bandings. The table was oriented in reverse Trendelenburg position (15°-20°) and tilted to patient’s right side (5°-8°). The patient was then prepped to widely expose the abdomen and a pneumoperitoneum was created and maintained at 10 mmHg. A total of five ports were used: four robotic ports of 8 mm in size and one laparoscopic port of 12 mm in size (to be used by the assistant at the table and accepting an endoscopic stapler), with the da Vinci Xi; three robotic ports of 8 mm in size, one laparoscopic port of 11 mm in size (for the robotic camera) and one laparoscopic port of 12 mm in size, with the da Vinci Si. The optic port was placed just above or below the umbilicus, depending on individual abdominal configuration. The 12 mm port was placed along the right pararectal line[14,15][Figure1].
Figure 1. Ports placement. A: ports placement for the robotic system da Vinci Xi; B: ports placement for the robotic system da Vinci Si. RA: robotic arm; A: laparoscopic assistant port; OP: optic port
The procedure started by opening the reflection of colon and omentum and mobilizing the left colonic flexure. Next, the peritoneum along the inferior margin of the pancreas was incised and the body-tail of the pancreas was mobilized on the posterior plane. The splenic vein was identified close to the inferior border of the body of the pancreas and clearly visualized before proceeding with further dissections. The common hepatic artery was identified next, as it provided a key landmark for safe division of the pancreas once a tunnel was created behind the pancreatic neck. The origin of the splenic artery was also conveniently identified ad encircled with a vessel loop for clear visualization during further dissections and to be available for crossclamping in case of bleeding. The pancreatic neck was divided using either an endoscopic stapler or a combination of dissection devices (with selective ligature of the pancreatic duct and subsequent oversewing of the pancreatic stump). With the splenic vessels in clear view dissection proceeded medial to lateral. Small vein branches were fixed by either energy devices or ligature. Small splenic arteries were all ligated or suture-ligated. Although systematic lymphadenectomy was not performed, lymph nodes around the splenic vessels were removed to permit prognostic stratification in case of unexpected post-operative diagnosis of a malignant tumor. At the end of the procedure the round ligament was mobilized and placed to cover naked vessels close to the pancreatic stump. A drain was left near the pancreatic stump[16].
When the splenic vessels could not be preserved, or were injured during dissection, before taking the decision to proceed with a Warshaw procedure or with splenectomy, resection and reconstruction or repair were taken into consideration.
Outcome measures
All post-operative events were recorded and classified according to standard outcome metrics[17-19]. Post-operative pancreatic fistula (POPF) was considered clinically relevant when graded B or C according to the definition of the international study group (ISGPF)[17]. Complications graded ≥ III in the Dindo-Clavien classification were considered severe[20]. The overall burden of complications was denied using the comprehensive complication index[21]. Post-operative mortality was considered as any death occurring during the first 90 days after surgery or during the initial hospital stay if longer.
Results
During the study period 54 patients were selected for possible RA-SPDP. The baseline characteristics of these patients are summarized in Table 1.
Baseline characteristics of 54 candidates for RA-SPDP
| Study population | |
|---|---|
| Number of patients (%) | 54 (100%) |
| Median age, years (IQR) | 60 (46.5-66) |
| Gender, male (%) | 14 (25.9%) |
| Median BMI, kg/m2 (IQR) | 24.1 (21.6-26.3) |
| Comorbidity (%) | 33 (61.1%) |
| Pre-operative symptoms (%) | 20 (37.3%) |
| Prior abdominal surgery (%) | 28 (51.8%) |
| Median ASA score (IQR) | 2 (2-3) |
There was no conversion to open or laparoscopic surgery. The spleen and the splenic vessels were preserved in 52 of 54 patients scheduled for RA-SPDP (96.3%). In three patients the splenic vessels had to be reconstructed to avoid a Warshaw procedure or a splenectomy. There were two elective reconstructions, caused by difficult detachment of the splenic vessels from the tumor, and one urgent reconstruction due to injury to the splenic vein. Overall, one splenic artery was reconstructed by end-to-end anastomosis and two splenic veins were reconstructed using an autologous interposition graft. A summary of intraoperative outcome measures is provided in Table 2.
Intra-operative outcome measures
| Result | |
|---|---|
| Median operative time, min (IQR) | 260 (231.3-360) |
| Median estimated blood loss, mL (IQR) | 150 (100-150) |
| Patients receiving blood transfusion, n (%) | 4 (7.4%) |
| Median number of blood units transfused per patient (IQR) | 1 (1-1) |
| Conversion, n (%) | 0 (0%) |
| Pancreatic stump closure: stapled, n (%) | 6 (11.1%) |
| Pancreatic stump closure: oversewn, n (%) | 48 (88.9%) |
A summary of the main post-operative outcome measures is provided in Table 3.
Post-operative outcome measures
| Result | |
|---|---|
| Median length of hospital stay (days) (IQR) | 10 (8-13) |
| Median CCI, n (IQR) | 20.9 (0-20.9) |
| Postoperative complications, n (%) | |
| Clavien-Dindo Grade 0 | 21 (38.9%) |
| Clavien-Dindo Grade I-II | 32 (59.3%) |
| Clavien-Dindo Grade III-IV | 1 (1.8%) |
| Post-operative blood transfusions, n (%) | 5 (9.2%) |
| POPF, n (%) | 27 (50%) |
| Grade BL, n (%) | 8 (14.8%) |
| Grade B, n (%) | 19 (35.2%) |
| Grade C, n (%) | 0 |
| Clinically relevant POPF, n (%) | 19 (35.2%) |
| PPH, n (%) | 1 (1.8%) |
| Grade A, n (%) | 0 |
| Grade B, n (%) | 1 (1.8%) |
| Grade C, n (%) | 0 |
| DGE, n (%) | 3 (5.5%) |
| Grade A, n (%) | 1 (1.8%) |
| Grade B, n (%) | 2 (3.7%) |
| Grade C, n (%) | 0 |
| Reoperation, n (%) | 2 (3.7%) |
| Readmission, n (%) | 4 (7.4%) |
Some results are worth to be noted. First, only two patients developed severe post-operative complications. Both required repeat surgery to address bleeding and splenic infraction, respectively. The first patient was re-operated during the initial hospital stay, the bleeding was controlled, and the spleen was preserved. The second patient was re-operated at the time of hospital readmission. Overall, the median Comprehensive Complication Index was 20.9 (IQR: 0-20.9). Second, there were no grade C POPF, despite grade B POPF was observed in 19 patients (35.2%). Third, four patients were readmitted (7.4%).
Tumor types are reported in Table 4.
Histology of resected pancreatic tumors
| Tumor types | Number (%) |
|---|---|
| IPMN, n (%) | 10 (18.5%) |
| Malignant-IPMN, n (%) | 2 (3.7%) |
| MCN, n (%) | 10 (18.5%) |
| Malignant-MCN, n (%) | 1 (1.8%) |
| SCA, n (%) | 17 (31.5%) |
| RCC metastases, n (%) | 1 (1.8%) |
| NET, n (%) | 9 (16.6%) |
| NEC, n (%) | 4 (7.4%) |
Median tumor size was 26 mm IQR: (20-40). Excluding a patient with known diagnosis of metastasis from renal cell carcinoma, 53 patients were scheduled for RA-SPDP for tumors presumed to be benign, or not overtly malignant. Malignancy was instead discovered in 7 patients (13.2%) [Table 5].
Detailed histology of malignancies
| Tumor types | Histotype | T | n | Grading | Ki67 (%) |
|---|---|---|---|---|---|
| Malignant-IPMN | |||||
| Branch duct | Pancreatobiliary, with foci of invasive adenocarcinoma | 1 | 0 | - | - |
| Branch duct | Pancreatobiliary, with in-situ adenocarcinoma | - | - | - | - |
| Malignant-MCN | |||||
| In-situ cystoadenocarcinoma | - | - | - | - | |
| NEC | |||||
| 1 | - | 3 | 1 | 1 | 1 |
| 2 | - | 3 | 1 | 2 | 5 |
| 3 | - | 2 | 0 | 2 | 8 |
| 4 | - | 2 | 1 | 2 | 7 |
There were no cases of margin positivity (at 1 mm), in the group of patients with malignant tumors, and the mean number of examined lymph nodes was 13.2 ± 12.3. Lymph nodes were positive in 3 patients with neuroendocrine cancer. Among a group of 10 patients with intraductal mucinous papillary tumors (IPMN) and worrisome features[22], two were found to be overtly malignant and of pancreatobiliary type. In one of these patients the tumor was in-situ. In the other patient showed focal infiltration of pancreatic parenchyma (T1). This patient was re-operated three months after the initial surgery to receive splenectomy and completion of the procedure according to oncologic principles. Repeat surgery was performed again using a robotic approach. Additional tissues removed showed no residual malignant growths either in the segment pancreatic body left behind at the initial surgery or in 22 retrieved lymph nodes.
After a mean follow-up period of 48.6 ± 30.6 months no patient developed evidence of either tumor recurrence (for those with a malignant histology) or splenic vein thrombosis (excluding the patient who required splenectomy due to splenic infarction).
Discussion
Minimally invasive distal pancreatectomy is gaining momentum[23]. The technique for minimally invasive distal pancreatectomy is indeed less demanding than the one required for minimally invasive pancreatoduodenectomy, so that virtually all pancreatic pancreatic surgeons, and most general surgeons, can perform this procedure safely in the absence of hostile anatomy and/or advanced tumor stage. However, minimally invasive distal pancreatectomy is a quite rare operation, even at high volume centers[24], requiring careful patient selection[25] and the ability to fully master minimally invasive techniques[26]. Patient selection is required to avoid either unnecessary procedures in patients with benign lesions with limited risk of malignant degeneration[27], or to plan the most appropriate therapeutic strategy for patients with pancreatic cancer[28]. Mastering of surgical technique is required to adapt the procedure to tumor type, and to face complex intraoperative scenarios that are sometimes unexpected. Minimally invasive spleen preserving distal pancreatectomy is the perfect example of this paradigm as it requires both extra careful patient selection and fine surgical technique. The robot, in competent hands, is a useful tool to improve surgical precision and maximize the rate of spleen preservation. However, it cannot surrogate for competency and basic surgical technique. Preservation of the spleen along with the splenic vessels requires fine dissection and the ability to safely manage small pancreatic vessels. The learning curve for this procedure has not been defined but is expected to be longer than the one reported for distal pancreatectomy with en-bloc splenectomy[15]. So far, unfortunately, there is also no validated program for systematic training of novices. While International Societies are working on these programs, background experience with other robotic procedures, video review, procedure observation, on-site proctoring (possibly using the dual console), and careful selection of patients are all believe to be important to permit safe implementation of a program for RA-SPDP.
Our series confirms that RA-SPDP is feasible in most patients, when selected appropriately, with a high probability of spleen preservation and a low incidence of severe complications. Admittedly, we have approximately 20 years of experience in minimally invasive distal pancreatectomy[29], we have performed approximately 400 robotic pancreatectomies, and we treat hundreds of new patients each year with surgical diseases of the pancreas and the periampullary region.
While robotic assistance is certainly associated with increased costs and longer operative times[30-32], there is no doubt that the use of da Vinci Surgical System enhances surgeon’s ability to preserve the spleen during distal pancreatectomy[33,34]. In this respect we believe that the robot is particularly useful when the Kimura technique is adopted as it allows safe dissection and preservation of splenic vessels. Although the Warshaw procedure can be considered when the splenic vessels cannot be preserved, the overall results of this operation are inferior to those of the Kimura procedure[35] making preservation of the splenic vessels preferable, whenever feasible. In this respect our experience is quite unique as we had never to adopt the Warshaw procedure, that was instead adopted in 28% to 50% of the patients in other robotic series[36,37].
We have previously reported that in our hands the risk of unintentional resection of a serous cystadenoma not causing symptoms was 2.1%[38].
We wish here to underscore that asymptomatic patients with a known diagnosis of serous cystadenoma should not undergo resection regardless of the size of the tumor. We wish also to emphasize that availability of robotic technology and ability to perform a minimally invasive procedure sparing the spleen is not a reason to expand indications to resection. The seemingly high rate of resected serous cystadenomas should therefore be read in the light of the high selection applied to patients reported herein to include only patients with presumably benign tumors. If the same figures were put in the context of our general activity, the rate of resected serous cystadenomas would not exceed 5%.
Our results underscore the importance of patients’ selection, not only from the perspective of spleen preservation but also, and perhaps even more importantly, because of the risk of missed malignancy. In our series we found that tumors initially thought to be pre-malignant were instead already overtly malignant in 7 patients (13.2%). Some of these tumors were either in situ or low-grade, so that RA-SPDP could be adequate anyway. On the other hand, we had a case of invasive pancreaticobiliary IPMN and four cases of neuroendocrine carcinoma with lymph nodes metastasis. In these patients the oncologic issue is not to have left the spleen behind, but having spared the splenic vessels and, possibly, having not performed an adequate lymphadenectomy. Indeed, spleen preservation, but using the Warshaw technique, was recently proposed even for pancreatic cancer considering that lymph node metastasis in the splenic hilum are exceedingly rare[39]. Sparing the splenic vessels, instead, could leave behind microscopic tumor residual (R1 resection). Although this was not the case in our series, even at the level of vascular beds, the risk is real. Our policy of systematic lymph node clearance around splenic vessels permitted to have a clearer picture of the tumor stage, so that we could decide how to manage these cases based on sound data. However, if the malignant tumor is in the body of the pancreas, several lymph node stations are not cleared (such as station number 9) that could harbor metastatic lymph nodes.
In this series we have rarely employed a stapler to divide the pancreas. We have rather preferred to divide the gland sharply while identifying and ligating selectively the pancreatic duct. There is no agreement on the ideal technique for pancreatic transection and closure during distal pancreatectomy[1]. During the first part of our experience, after sharp division of the pancreas the duct was selectively ligated, and the stump closed with interrupted sutures. Later, thanks to the availability of robotized laparoscopic staplers (namely the SigniaTM power handle and the iDriveTM - Minneapolis, Medtronic, Covidien) we converted to the use of these devices. The stapler was handled and fired by the assistant at the table. The size of the cartridge was decided based on the thickness of the pancreatic parenchyma. In most patients a purple cartridge reinforced with a bioabsorbable line reinforcement.
Robotic assistance allows to do this as easily as in open surgery, especially when the pancreatic body is partially spared and the pancreas is not divided at the neck, where the stapler should be ideally applied to achieve the best results. While in malignant tumors there is no good reason to spare a portion of the pancreatic body, in benign or pre-malignant tumors parenchymal sparing distal pancreatectomy can be conveniently adopted to reduce the metabolic and digestive consequences of partial pancreatectomy. The literature shows that incidence and severity of POPF are similar irrespective of which surgical technique is used[40]. Therefore, it is not surprising that robotic assistance does not reduce the occurrence of this complication.
In conclusion, robotic assistance, although not essential, is important to maximize the probability of spleen preservation along with the splenic vessels. RA-SPDP is also associated with a low conversion rate and a limited incidence of severe post-operative complications.
Declarations
Authors’ contributionsConception and design: Kauffmann EF, Boggi U
Provision of study materials or patients: Kauffmann EF, Napoli N, Menonna F, Genovese V, Cacace C, Gianfaldoni C, Vistoli F, Boggi U
Collection and assembly of data: Kauffmann EF, Napoli N, Menonna F, Genovese V, Cacace C, Gianfaldoni C, Vistoli F, Amorese G, Boggi U
Data analysis and interpretation: Kauffmann EF, Menonna F, Boggi U
Manuscript writing: Kauffmann EF, Napoli N, Menonna F, Genovese V, Cacace C, Vistoli F, Amorese G, Boggi U
Final approval of manuscript: Kauffmann EF, Napoli N, Menonna F, Genovese V, Cacace C, Gianfaldoni C, Vistoli F, Amorese G, Boggi U
Availability of data and materialsData are stored in a database at University of Pisa.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThe study was approved by the local ethic committee.
Consent for publicationThe informed consent of the person in the video was obtained.
Copyright© The Author(s) 2020.
REFERENCES
1. Asbun HJ, Moekotte AL, Vissers FL, Kunzler F, Cipriani F, et al. The miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg 2020;271:1-14.
2. Briggs CD, Mann CD, Irving GRB, Neal CP, Peterson M, et al. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg 2009;13:1129-37.
3. Leonard AS, Giebink GS, Baesl TJ, Krivit W. The overwhelming postsplenectomy sepsis problem. World J Surg 1980;4:423-32.
4. Malangoni MA, Dillon LD, Klamer TW, Condon RE. Factors influencing the risk of early and late serious infection in adults after splenectomy for trauma. Surgery 1984;96:775-83.
5. Buzelé R, Barbier L, Sauvanet A, Fantin B. Medical complications following splenectomy. J Visc Surg 2016;153:277-86.
6. Rottenstreich A, Kleinstern G, Spectre G, Da’as N, Ziv E, et al. Thromboembolic events following splenectomy: risk factors, prevention, management and outcomes. World J Surg 2018;42:675-81.
7. Kimura W, Yano M, Sugawara S, Okazaki S, Sato T, et al. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein: techniques and its significance. J Hepatobiliary Pancreat Sci 2010;17:813-23.
8. Kimura W, Inoue T, Futakawa N, Shinkai H, Han I, et al. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein. Surgery 1996;120:885-90.
10. Freschi C, Ferrari V, Melfi F, Ferrari M, Mosca F, et al. Technical review of the da Vinci surgical telemanipulator. Int J Med Robot Comput Assist Surg 2013;9:396-406.
11. Moorthy K, Munz Y, Dosis A, Hernandez J, Martin S, et al. Dexterity enhancement with robotic surgery. Surg Endosc Other Interv Tech 2004;18:790-5.
12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9.
13. Keats AS. The ASA classification of physical status - a recapitulation. Anesthesiology 1978;49:233-6.
14. Napoli N, Kauffmann EF, Menonna F, Iacopi S, Cacace C, et al. Robot-assisted radical antegrade modular pancreatosplenectomy including resection and reconstruction of the spleno-mesenteric junction. J Vis Exp 2020; doi: 10.3791/60370.
15. Napoli N, Kauffmann EF, Perrone VG, Miccoli M, Brozzetti S, et al. The learning curve in robotic distal pancreatectomy. Updates Surg 2015;67:257-64.
16. Kauffmann EF, Napoli N, Menonna F, Cacace C, Genovese V, et al. Robot-assisted spleen preserving distal pancreatectomy: case report. Ann Laparosc Endosc Surg 2020; doi: 10.21037/ales.2020.03.14.
17. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surg 2017;161:584-91.
18. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the international study group of pancreatic surgery (ISGPS). Surgery 2007;142:761-8.
19. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, et al. Postpancreatectomy hemorrhage (PPH)-an international study group of pancreatic surgery (ISGPS) definition. Surgery 2007;142:20-5.
20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13.
21. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1-7.
22. Del Chiaro M, Besselink MG, Scholten L, Bruno MJ, Cahen DL, et al. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018;67:789-804.
23. Capretti G, Boggi U, Salvia R, Belli G, Coppola R, et al. Application of minimally invasive pancreatic surgery: an Italian survey. Updates Surg 2019;71:97-103.
24. Balzano G, Bissolati M, Boggi U, Bassi C, Zerbi A, et al. A multicenter survey on distal pancreatectomy in Italy: results of minimally invasive technique and variability of perioperative pathways. Updates Surg 2014;66:253-63.
25. Klompmaker S, Van Zoggel D, Watkins AA, Eskander MF, Tseng JF, et al. Nationwide evaluation of patient selection for minimally invasive distal pancreatectomy using American College of Surgeons’ National Quality Improvement Program. Ann Surg 2017;266:1055-61.
26. Ohtsuka T, Ban D, Nakamura Y, Nagakawa Y, Tanabe M, et al. Difficulty scoring system in laparoscopic distal pancreatectomy. J Hepatobiliary Pancreat Sci 2018;25:489-97.
27. Sharib JM, Fonseca AL, Swords DS, Jaradeh K, Bracci PM, et al. Surgical overtreatment of pancreatic intraductal papillary mucinous neoplasms: do the 2017 international consensus guidelines improve clinical decision making? Surg (United States) 2018;164:1178-84.
28. Balzano G, Capretti G, Callea G, Cantù E, Carle F, et al. Overuse of surgery in patients with pancreatic cancer. A nationwide analysis in Italy. HPB 2016;18:470-8.
29. Pietrabissa A, Moretto C, Boggi U, Di Candio G, Mosca F. Laparoscopic distal pancreatomy: Are we ready for a standardized technique? Semin Laparosc Surg 2004;11:179-83.
30. Hong S, Song KB, Madkhali AA, Hwang K, Yoo D, et al. Robotic versus laparoscopic distal pancreatectomy for left-sided pancreatic tumors: a single surgeon’s experience of 228 consecutive cases. Surg Endosc 2020;34:2465-73.
31. Chen S, Zhan Q, Chen JZ, Jin JB, Deng XX, et al. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc 2015;29:3507-18.
32. Waters JA, Canal DF, Wiebke EA, Dumas RP, Beane JD, et al. Robotic distal pancreatectomy: cost effective? Surgery 2010;148:814-23.
33. Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32.
34. Huang B, Feng L, Zhao J. Systematic review and meta-analysis of robotic versus laparoscopic distal pancreatectomy for benign and malignant pancreatic lesions. Surg Endosc 2016;30:4078-85.
35. Li BQ, Qiao YX, Li J, Yang WQ, Guo JC. Preservation or ligation of splenic vessels during spleen-preserving distal pancreatectomy: a meta-analysis. J Investig Surg 2019;32:654-69.
36. Esposito A, Casetti L, De Pastena M, Ramera M, Montagnini G, et al. Robotic spleen-preserving distal pancreatectomy: the Verona experience. Updates Surg 2020; doi: 10.1007/s13304-020-00731-8.
37. Eckhardt S, Schicker C, Maurer E, Fendrich V, Bartsch DK. Robotic-assisted approach improves vessel preservation in spleen-preserving distal pancreatectomy. Dig Surg 2016;33:406-13.
38. Lombardo C, Iacopi S, Menonna F, Napoli N, Kauffmann E, et al. Incidence and reasons of pancreatic resection in patients with asymptomatic serous cystadenoma. Pancreatology 2018. S1424-3903:30606-9
39. Kawaguchi Y, Fuks D, Nomi T, Levard H, Gayet B. Laparoscopic distal pancreatectomy employing radical en bloc procedure for adenocarcinoma: technical details and outcomes. Surg 2015;157:1106-12.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Kauffmann EF, Napoli N, Menonna F, Genovese V, Cacace C, Gianfaldoni C, Vistoli F, Amorese G, Boggi U. Robot-assisted spleen preserving distal pancreatectomy (RA-SPDP): a single center experience. Mini-invasive Surg 2020;4:54. http://dx.doi.org/10.20517/2574-1225.2020.46
AMA Style
Kauffmann EF, Napoli N, Menonna F, Genovese V, Cacace C, Gianfaldoni C, Vistoli F, Amorese G, Boggi U. Robot-assisted spleen preserving distal pancreatectomy (RA-SPDP): a single center experience. Mini-invasive Surgery. 2020; 4: 54. http://dx.doi.org/10.20517/2574-1225.2020.46
Chicago/Turabian Style
Kauffmann, Emanuele Federico, Niccolò Napoli, Francesca Menonna, Valerio Genovese, Concetta Cacace, Cesare Gianfaldoni, Fabio Vistoli, Gabriella Amorese, Ugo Boggi. 2020. "Robot-assisted spleen preserving distal pancreatectomy (RA-SPDP): a single center experience" Mini-invasive Surgery. 4: 54. http://dx.doi.org/10.20517/2574-1225.2020.46
ACS Style
Kauffmann, EF.; Napoli N.; Menonna F.; Genovese V.; Cacace C.; Gianfaldoni C.; Vistoli F.; Amorese G.; Boggi U. Robot-assisted spleen preserving distal pancreatectomy (RA-SPDP): a single center experience. Mini-invasive. Surg. 2020, 4, 54. http://dx.doi.org/10.20517/2574-1225.2020.46
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 9 clicks
Cite This Article 9 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.