Nanomaterial-based hydrogels for coronary interventions: a mini review
Abstract
Myocardial infarction (MI) has become a major health concern these days. Elevated levels of cholesterol due to improper diet cause severe damage to human health, resulting in the narrowing of blood vessels leading to MI. Different approaches have been used based on surgical and non-surgical treatments for these blockages to cure MI. In this regard, injectable and non-injectable hydrogel-based percutaneous coronary intervention has shown promising applicability for the treatment of cardiac damage and its repair. In this report, we summarize a few hydrogels based on natural polymers such as chitosan, alginate, polyethylene glycol and extracellular matrices to be used for percutaneous coronary intervention in the treatment of MI. Their structure, biological properties and biocompatibilities are discussed, and their existing challenges are also detailed. In addition, the probable solutions to overcome certain set backs are also highlighted.
Keywords
Introduction
The heart functions regularly to recirculate the blood to the whole body. In general, the main function of the heart is to pump the oxygenated blood throughout the body. A breakdown in the functioning of the heart causes the irregular supply of oxygen to the organs and, consequently, can cause severe life-threatening effects such as heart failure, organ collapse and nerve damage, as well as the malfunction of various organs. In fact, cardiovascular diseases have been major fatal causes these days. Although recent advances in cardiac tissue engineering (CTE) such as stem cell therapy, artificial tissues and scaffold-based systems have emerged as powerful techniques for the treatment of coronary diseases, yet the developments of 3D printed scaffolds for the speedy treatment of the cardiac tissues are the demand of new era[1]. The improper flow of blood may result in myocardial infarction (MI), i.e., heart attack, simultaneously causing damage to heart cells. This condition usually occurs due to blockage in one or more of the coronary arteries. The situation arises due to the accrual of fats and cholesterol in and on the artery wall known as plaque, which restricts blood flow. Thrombosis is mostly caused by the rupture of plaque, which is explained as the structural defect or gap in the fibrous cap. This exposes the highly thrombogenic core to the blood[2]. The accumulation of these fats and cholesterol is known as atherosclerosis[3].
Percutaneous coronary intervention (PCI) is one of the most used non-surgical procedure for the treatment of atherosclerosis. In brief, a thin flexible tube known as a catheter is used to place a small stent (structure) in the heart vessels to open up the blood capillaries, when narrowed by the plaque[4]. Various materials have been utilized as a PCI tool for the treatment of MI. Soft material based hydrogels are being used in various forms in CTE[5-7]. This complex research area is being explored by various interdisciplinary approaches involving material scientists, cell biologists, chemical biologists and nanotechnologists. Among these approaches, nanotechnology has played an extensive role in the biomedical section due to the tunable surface and material properties exploited for PCI. The surface-to-volume ratio, surface charge, and integration with the cells and proteins make nanomaterials highly effective in various fields of biomedical science, including CTE. The wide biomedical applications of nanotechnology include but are not limited to drug delivery[8,9], tissue engineering[10,11], hyperthermia[12,13], and nanoantibiotics[14-17].
Various nanomaterials have also been utilized for the designing of PCI, and are being explored for their practical applicability. These nanomaterials possess unique mechanical properties for their applications in PCI for the treatment of MI. A few of the most studied materials used for PCI are alginate[7], chitosan[18], polyethylene glycol (PEG)[19] and extracellular matrix (ECM)[20]. In this review, we mostly focus on these four materials as hydrogels for PCI applications. We discuss the structure, biochemical interactions, and applications of these materials for PCI referring to a few of the recent studies. Additionally, we discuss the future prospects of these materials to be utilized for CTE as well as in the treatment of the MI.
Hydrogel-based coronary interventions
Hydrogels are chemically or physically cross-linked hydrophilic polymers, which possess effective mechanical as well as the chemical properties. These hydrogels are usually capable of absorbing biological fluids many times their weight, making them suitable for various biomedical applications. However, the major issue with hydrogels remains their toxicity to biological system. The residual monomer, cross-linker and catalysts cause the toxicity after the degradation of hydrogels[21]. As discussed above, various material-based hydrogels have been utilized for applications in PCI. PCI-based strategies may help damaged cardiac tissues to recover; however, severe cases require the implantation of ventricular assist devices, creating an invasive method for the treatment. The advancements in this field are ongoing with interdisciplinary approaches for the PCI-based treatments. A few of the recent advances using alginate-, ECM- and PEG-based hydrogels and their salient features are listed in Table 1.
Recent advances in hydrogel-based PCIs in cardiac repair
| S. N. | Materials | Major components | Salient features | Remarks | Ref. |
|---|---|---|---|---|---|
| 1. | Alginate dialdehyde-gelatin hydrogel | Alginate and gelatin | 3D orienting of cell-laden hydrogel
Homogenous cell distribution High cell viability | Suitable for 3D printing in cardiac tissue engineering | [22] |
| 2. | VentriGel | ECM from decellularized porcine myocardium | A first-in-man clinical trial of ECM-hydrogel
Safe and feasible in post-MI patients with left ventricular dysfunction Improvements in left ventricular remodeling > 1 year and vice versa for < 1 year of treatment | Efficient for the treatment of post-early and -late MI | [23] |
| 3. | Collagen-based hydrogel | Transglutaminase cross-linked gelatin | Stem cell-based therapy for ischemic heart disease
Improved retention and cardioprotection Combination therapy may protect against cardiac injury after MI | Dual functionality, suitable for the treatment of MI and cardiac repair | [6] |
| 4. | Thrombin-coagulated fibrin hydrogels | Decellularized ECM from porcine ventricular tissue and fibrinogen | Inclusion of cells due to thrombin
3D embedding enhanced cellular differentiation Recovery, frequency, synchrony and spontaneous beating | Suitable for cardiac cell differentiation | [20] |
| 5. | Alginate/ECM hydrogel | ECM from porcine heart into alginate | Enhanced rheological and mechanical properties
> 80% viability with > 100% metabolic activity Non-invasive delivery | Cell-free treatment of MI | [24] |
| 6. | PEG-based injectable hydrogels | Triblock copolymers (PDEGMA-b-PPEGMA-b-PDEGMA) | A triblock polymer- formed gel
Reversible sol-gel transformation 20 wt% formed strong gel in 5 seconds | Suitable as a scaffold for tissue engineering | [25] |
| 7. | Polydopamine-containing hydrogel membrane coating over the metallic stent | Polydopamine-containing hydrogel membrane- and acrylamide | Stable coating for a non-invasive approach
Improved HUVEC viability/proliferation and suppressed SMC viability Acrylamide enhanced mechanical strength | Non-invasive treatment of MI due to blockage | [26] |
| 8. | H2S releasing peptide hydrogel | Peptides FBA-IAVEE and FBA-IAVEEEE | Inhibited proliferation and migration of VSMCs
Reduced intimal hyperplasia Proliferation of human umbilical endothelial cells | Suitable as a coating material for stents | [27] |
| 9. | Hyaluronic acid hydrogel | Hyaluronic acid | Sustained miRNA-302 delivery by hydrogels
Local clonal proliferation at injection within 2 weeks Decreased cardiac end diastolic and end-systolic volumes, with improved ejection fraction and fractional shortening | miRNA-based therapy for cardiac tissue engineering | [28] |
| 10. | Silk fibroin microsphere-based alginate hydrogel | Alginate containing silk fibroin hydrogels | Sustained delivery of insulin-like growth factor 1 via hydrogel
Reduction in infarct size within 28 days Improved cardiac function | Suitable for cardiac tissue engineering | [29] |
The stiffness of materials, bioactivity, and biodegradability are the key factors that play an important role in the selection of hydrogels for PCI[30]. Nanotechnology in this regard provides an upper hand to the researchers to tune these properties by controlling the size, structure and morphology of the materials. However, each of these properties is crucial in the selection and rejection of the stent, yet the inherent properties of the material control the desired reactions. For example, the cross-linking of the materials controls the stiffness of the hydrogels[19]. In addition, the swelling and the degradation behavior are also important for the working efficiency as well as the acceptance/rejection of the hydrogels as stents[21]. Furthermore, various modifications have also been made on hydrogels for creating multi-functionality such as stent materials with imaging properties to track the exact location and the site of action[31]. Similarly, various drug delivery applications of hydrogel-based materials have also been utilized in the treatment of the MI. Hence, the tunable properties for the site-specific applications make hydrogels materials very specific in the design as well as the applications. Recent developments using chitosan-, alginate-, PEG and ECM-based hydrogels for the treatment of MI are discussed in the upcoming sections.
Chitosan-based hydrogels for PCI
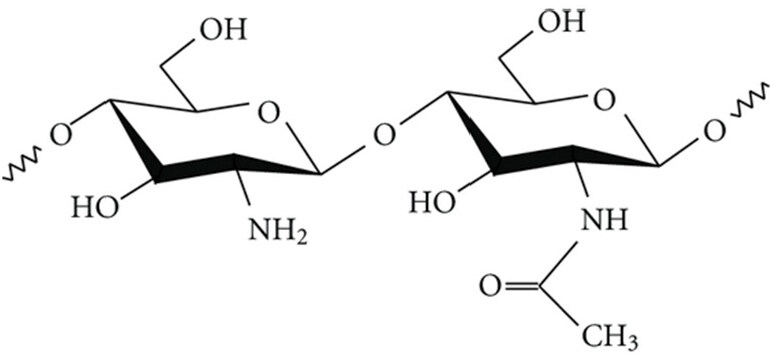
Chitosan is one of the most studied materials for the treatment of MI using PCI. Its cross-linking properties make it a promising candidate for creating 2D and 3D stents to be used for PCI. Chitin and chitosan (deacetylated derivative of chitin) are natural polymers and found abundant in nature (approx. 1000 t/year). They are made up of randomly distributed β-(1-4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit)[32] as shown in Figure 1[33]. Most of their properties such as bioactivity, biodegradability, antibacterial activity and cellular adhesion depend on the degree of deacetylation and molecular weight[9]. Besides, the amine groups present in chitosan provide the advantage of interacting with the cells as well as the cell adhesion proteins[18]. However, cellular and enzymatic rejection of chitosan-based hydrogels is the major lag restricting their practical applications. Hence, cross-linking plays an important role in the biological response of chitosan.
Figure 1. Chemical structure of chitosan, comprising N-acetyl-D-glucosamine (right) and D-glucosamine (left) units. Adapted with permission from Andrade et al.[33]
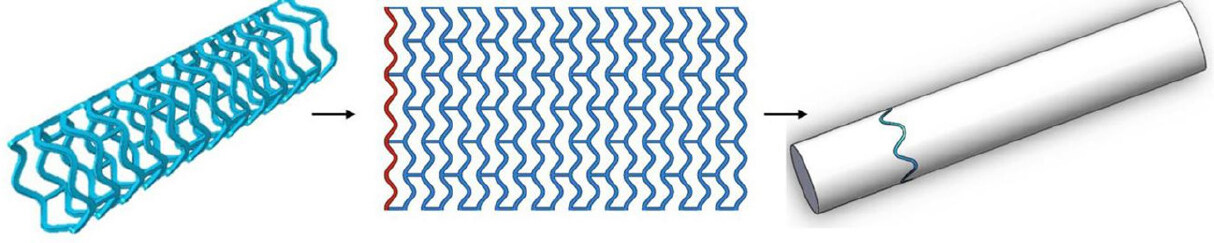
Various approaches for the use of chitosan as a hydrogel for cardiac treatment, especially as a stent, have been made because of its tunable stiffness, wettability and swelling properties[9,18]. It is well known that sulfated chitosan enhances the bioactivity of the material[34]. In a recent study, Qiu et al.[35] designed a 3D-printed bioresorbable stent using polycaprolactone (PCL), surface modified with sulfated chitosan. Chlorosulfuric acid (HClSO3) was used to sulfonate chitosan at 70 ºC. A polymeric tabular stent (diameter × length: 3 mm × 10 mm) of PCL was 3D-printed using the electrospinning technique, as shown in Figure 2. The mechanical properties of PCL stents were not compromised after modification with sulfated chitosan. No displacement was observed up to 0.7 N of force in either PCL- or sulfated chitosan-modified stent. Enzymatic degradation (wt%) was found to be 16% and 7% with and without lysozyme, respectively, after 60 days. These features indicated the suitability of the sulfated chitosan-modified stent for PCI applications.
Figure 2. 3D-printing trajectory strategy of polycaprolactone stent. Adapted with permission from Qiu et al.[35]
In another study, Si et al.[18] made a biopolymeric conductive hydrogel with conductive nano-dots. For this purpose, the authors prepared a chitosan/collagen hydrogel and combined it with graphene quantum dots (CS/CG-GQDs). Later, the designed hydrogel was impregnated with human mesenchymal stem cells (hMSCs), which resulted in improved angiogenesis and consequently decreased the cardiomyocyte necrosis caused by the hydrogel. The addition of conductivity, as well as hMSCs decreasing the death rate of cardiomyocytes, and the addition of GQDs, healed the fibrosis by altering electrical conductivity, leading to the treatment of the cardiac tissues. A 3D porous network with pore size of 20 ± 3 μm and 32 ± 5 μm was respectively obtained for CS/CG and GQDs-CS/CG hydrogels. The designed hydrogel possessed enhanced cell survival rate and pro-angiogenic factors, making it suitable for CTE to treat acute MI. Both studies suggest that the addition of chitosan creates a stent free from various cellular and enzymatic rejection problems and rapid degradation, leading to a suitable stent-based angioplasty after acute MI.
Various other studies have been conducted using chitosan as hydrogel material for PCI. Chitosan, being highly bioactive, biocompatible and moderately biodegradable, has also been used as a coating material on various stents for the treatment of MI[35]. In such an approach, Lin et al.[36] coated polyvinyl alcohol (PVA) fibers with chitosan using the spray coating method, without sealing the meshes. PVA fibers were fashioned into braids using 16-spindle braider and cross-linked with glutaraldehyde to stabilize the interlacing points followed by spray coating with chitosan. Mesh sizes ranging from 0.20-0.35 mm2 with a membrane thickness of 0.27-0.41 μm were obtained. Chitosan coating was reported to improve compression resistance. It was found that the chitosan-coated PVA stents were suitable for use in PCI because of their higher bioactivity and cytocompatibility (80% cell viability).
A blending approach has also been attempted to enhance the biocompatibility as well as the biodegradation of the stent material. In a recent study, poly-lactic acid/chitosan nanofibers were electrospun and loaded with paclitaxel as a coating material for the prototype polymeric stent. A single-nozzle electrospinning approach was utilized to make the fibrous stent. The chitosan concentration was varied from 3-9 wt%, and the drug loading concentration wt was varied from 40-120 wt% to obtain an optimum composite fiber. The physical encapsulation of the drug in the polymeric matrix without any chemical bonding was reported. The samples with the 40% and 60% drug loading displayed controlled drug release. An increase in chitosan concentration provided more homogenous fibers with smaller mean diameter, yet agglomeration was observed after 5% chitosan. Excellent cell viability (> 90%) was observed up to 60% drug loading. However, a further increase in the drug concentration resulted in decreased cell viability. The cell viability was decreased to 50% at a drug concentration 80% due to the exceeding the cytotoxic limit[37]. Hence, these findings suggested that chitosan-containing stents are very effective for use in stents for PCI.
Alginate-based hydrogels for PCI
Alginate, a natural polymer, has also been studied as a potential material for cardiac stents in the treatment of MI. Alginate possesses moderate cross-linking properties demonstrating it as a suitable candidate for stent preparation. Alginates are linear copolymers and are mostly composed of (1-4)-linked α-L-guluronic (G) and β-D-mannuronic (M) residues as shown in Figure 3. The number of sequences depends on the isolation species, i.e., the organisms and tissues. The random sequences of these G and M residues intercalate to form the alternating region of the MG blocks. The rigid 6-membered sugar rings add the restricted rotation around the glycosidic linkage and provide stiffness to the alginate[38]. This indicates its suitability for stent applications, as stiffness similar to that of the artery is a required property of stent material for proper blood flow, restriction from mechanical damage and degradation.
Figure 3. Chemical structure of alginate. G and M refer to α-L-guluronic and β-D-mannuronic residues, respectively. Reprinted (adapted) with permission from Hecht et al.[38]. Copyright (2016), American Chemical Society
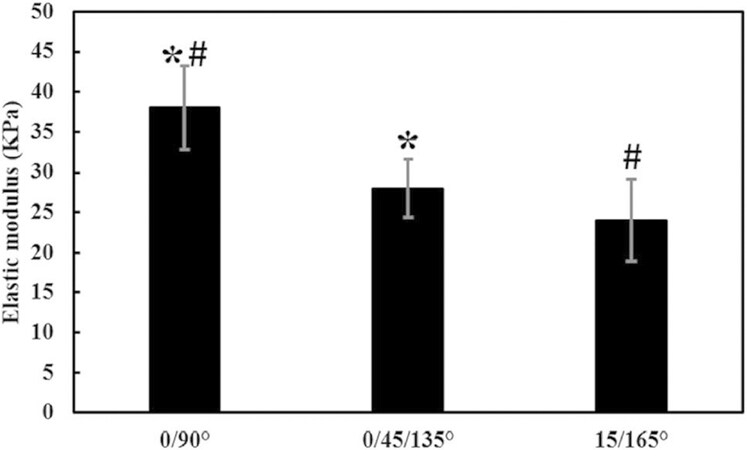
Alginate has been utilized for various biomedical applications because of its gelling properties and natural origin. Cross-linking is usually done by the diffusion method using Ca(II) or Na(I) ions making the polymer stiffer[39]. The inherent pre-requisite of cross-linking for the gel formation by alginate monomer makes it more effective and provides the competence to tune the stiffness for various applications. In addition, high mechanical and chemical stability, adequate swelling properties, narrow pore size distribution and pore size[40] make alginate a strong candidate for stent formation. However, its poor bioactivity and biocompatibility, as well as its stiffness equivalent to the surrounding tissues, need to be explored prior to its application. Various approaches have been explored to use alginate as stent material for the treatment of MI using PCI. Recently, You et al.[22] prepared an alginate dialdehyde-gelatin hydrogel bio-ink for 3D printing for the treatment of MI. The 3D bioplotter system was used for printing of the scaffolds. The authors claimed that 10% oxidation degree of 70 wt% alginate dialdehyde and 30 wt% gelatin concentration provided the best printability, making it suitable for the 3D printed scaffolds. The authors also seeded the living cells (EA.hy926) and demonstrated cell spreading as well as excellent cell viability up to 7 days. It was found that the above proportions provided the most homogenous cell distribution among the scaffolds with cell viability of > 90% after 7 days. In terms of mechanical properties, 0°/90° pattern showed the higher elastic modulus (~33 Pa) than that of 0°/45°/135° (~28 kPa) and 15°/165° (~24 kPa) patterns as shown in Figure 4. The scaffolds were found to be suitable for long-term application for CTE as well as the treatment of the MI. PCI using such bioinks provide a suitable stiffness, cell interaction and cell proliferation as well for biomedical applications. Similarly, Sack et al.[41] demonstrated that alginate hydrogel injections act as a suitable candidate in the treatment of MI as a left ventricular mid-wall constraint in swine. It was claimed that the hydrogel injection therapy moderated the elongation in sarcomere lengths from 1.78 ± 0.15 μm to 1.68 ± 0.10 μm after the treatment. In addition, systolic contractility (ejection fraction) was significantly enhanced from 34.7% ± 2.7% to 43.9% ± 2.8%. Moreover, the designed model showed realistic simulation with > 99% accuracy, when small myofiber strain in the nearby solidified hydrogel was kept at 13 mm away from the implant. These findings clearly showed that solidified alginate-based materials may mimic the mid-wall structure of the left ventricles, and can be used for various cardiac applications. Both of the latter studies showed the effective interaction of cardiac cells with alginate. Alginate hydrogels possess the required stiffness as well as the mechanical properties in comparison to cardiac cells, demonstrating them as one of the most suitable candidates for use in the treatment of MI.
Figure 4. Elastic modulus of 10% alginate dialdehyde-gelatin hydrogels (70-30 wt%) with three angular designs: (1) each layer adhered to the underlying layer at 90° (0/90°); (2) the second layer adhered to the underlying layer at 45° and the third layer adhered to the first layer at 135° (0/45/135°); and (3) second layer adhered to the first layer (15°) at 165° (15/165°) (#,*P < 0.05). Adapted with permission from You et al.[22]
In addition, there are various other properties that also make alginate a suitable candidate for use in the treatment of MI. Its non-toxicity to blood cells has been a keen objective for researchers. Various studies have been done on the blood cell toxicity of alginates. In this direction, Qi et al.[42] studied alginate oligosaccharide for CTE and demonstrated its effect on the human platelet aggregation. A concentration-dependent inhibition of human platelet aggregation, clot retraction and spreading was obtained for the alginate oligosaccharide in the concentration range of 0.1-1.0 mg/mL. Similarly, ATP release was found to be concentration-dependent and was induced by thrombin and collagen formation. Bleeding time was found to be 534 ± 62 s in vehicle control and 581 ± 60 s in mice with alginate pretreatment. These findings demonstrated the blood compatibility of the alginate and its plausible applications in the treatment of MI. In addition, the blends of alginate with various materials have also been explored to obtain the desired biocompatibility for the biomaterial-based treatment of MI. In this regard, Curley et al.[24] designed an injectable alginate/ECM hydrogel for the acellular treatment of MI. The storage modulus, compressive modulus and dynamic modulus for high G block alginate/ECM hybrid hydrogel at day 1 were found to be 1.6, 29 and 14 kPa, respectively. The excellent cell proliferation (> 85%) with metabolically active cells (> 100%) as compared to the control was obtained, proving the hybrid alginate-ECM system to be a suitable candidate for non-invasive treatment of MI. All these studies showed that alginate-based materials have excellent biological properties for CTE.
PEG-based hydrogels for PCI
PEG has been utilized for various biomedical applications because of their highly tunable size and orientations. PEG is a polyether compound based on its molecular weight. It is also known as polyethylene oxide or polyoxyethylene. PEG is considered a water-soluble, low immunogenic and biocompatible polymer. PEGylation expands the orientation as well as the size of the conjugated compounds, which consequently results in resistance to enzymatic digestion, making it suitable for various biomedical applications, including the treatment of MI[43]. PEG has various properties that makes it an ideal candidate to be used as hydrogel material in PCI. Additionally, its different solvent-based orientations provide a tunability for various applications. For example, in hexadecane, it carries a well described freely jointed chain structure, whereas in water, a deformation in the supra-structure within the polymer has been observed, resulting in entropic to enthalpic elasticity[44]. This restricts its water-based applicability in terms of mechanical properties and biocompatibility.
Various approaches have been examined to use PEG for PCI in the treatment of MI on the basis of its solvent-selective elasticity as well as water solubility. Recently, Boyacioglu et al.[45] studied the shape memory behavior of PEG plasticized Polylactic acid (PLA)/thermoplastic polyurethane (TPU) blends. The shape memory behavior was investigated as a function of PLA/TPU ratio, plasticizer molecular weight and programming conditions. The plasticization efficiency was found to decrease with increase in molecular weight of PEG. It was claimed that with an increase in TPU content, the recovery ratio between 40-55 °C was also increased. However, at 60 °C for 20/80 PLA/TPU blends, the maximum total recovery (> 80%) was obtained because of the strong elasticity of TPU. The shape memory values were found to be dependent on PEG molecular weight in a reverse order, and the blend was able to manage 245 kPa of stress, indicating its applicability for PCI in the treatment of MI.
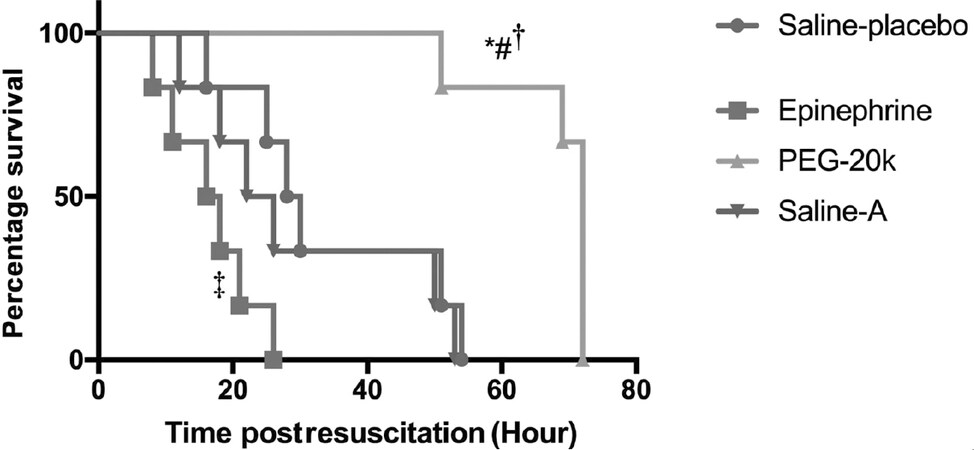
In another study, Lin et al.[46] prepared the PCL/PEG coated PVA biodegradable composite stents with a core-shell structure for various applications. The coated yarns were weft knitted into braids followed by thermal treatment, which resulted in a core-shell structure. In a typical process, the authors fitted the PVA yarns into a machine, followed by twisting, coating, and weft knitting. Different ratios (wt%) of PCL/PEG, i.e., 100/0, 90/10, 80/20, 70/30, 60/40, and 50/50 were melted and blended 5 times at various processing temperatures ranging from 70-100 °C at a step size of 10 °C. A coating machine was utilized to coat the PCL/PEG mixtures followed by heat treatment at 60 °C for 15 min to form the stents. The diameter of the composite stent was found to be 3 mm. The high PCL ratios, i.e., PCL/PEG 100:0 and 90:10 possessed the high porosities of 24.93% and 26.50%, respectively. Further increase in the PEG content from 20-30 wt% decreased porosity to 23.39% and 23.29%, respectively. This indicated that porosity increased up to 20% of PEG concentration and was decreased with further increase in concentration. This study clearly showed that porosity is a function of PCL/PEG concentrations. The synergistic effect on the thermal behavior of the composite was confirmed by the crystallization temperature ranging between that of PCL and PEG. The compressive strength of composite stents was found to be enhanced with an increase in PEG concentration up to 30% (6.15 N) and decreased with a further increment (4.5 N). The maximum cell viability was also observed at 30% PEG concentration, i.e., > 90%, and decreased with a further increment (~40%), indicating a correlation between mechanical properties, PEG concentration and cell viability as well. This study helps to understand the effects of PEG concentration on cell attachment as well as the mechanical properties required for the use of PEG for designing the stents. Ge et al.[47] examined the effects of aortic-infused PEG-20k during cardiopulmonary resuscitation on various cardiac functions. An increase in coronary perfusion pressure to the same extent as with epinephrine resulted in an improvement in the post-resuscitation myocardial and cerebral functions and inhibition of cardiac arrest. The in vivo studies showed that four rats survived in the PEG-20k groups, zero rats in the saline-placebo and only 1 rat survived after > 24 h in the epinephrine group, as shown in Figure 5. The studies explain that the cardiac functioning of the PEG-based material depends on its structure, molecular weight and orientation as well. Similarly, Aykar et al.[48] manufactured the self-standing microfluidic chip using PEG-diacrylate (PEGDA)-based hollow microvessels with inner dimensions of 15-73 μm. The macromer solutions were focused onto a single microchannel hydrodynamically and were subsequently solidified through photopolymerization. The emphasis was to mimic the arteries (0.1 mm to > 1 cm), arterioles (10-100 μm), capillaries (4-12 μm), venules (10-100 μm), and veins (0.1 mm to > 1 cm). The optimized wt% for a balance in mechanical strength and cytocompatibility was found to be 50% of PEGDA. All these studies have proven PEG as a special candidate with highly tunable cell response, mechanical strength, bioactivity and cell functioning as well. PEG can be tuned on the basis of its molecular weight, orientation and cross linking with other materials as well. PEG is mostly blended with existing materials such as PCL to synergistically enhance the desired properties for PCI in the treatment of MI.
Figure 5. Survival of rats; PEG-20k improves survival duration vs. PEG-20k with saline placebo vs. saline placebo with epinephrine. Adapted with permission from Ge et al.[47]. *P = 0.0022 vs. PEG-20k with saline placebo; #P = 0.0016 vs. PEG-20k with Saline-A; †P = 0.0005 vs. PEG-20k with epinephrine; ‡P = 0.012 vs. saline placebo with epinephrine. PEG: polyethylene glycol
ECM hydrogels in cardiac tissue repair
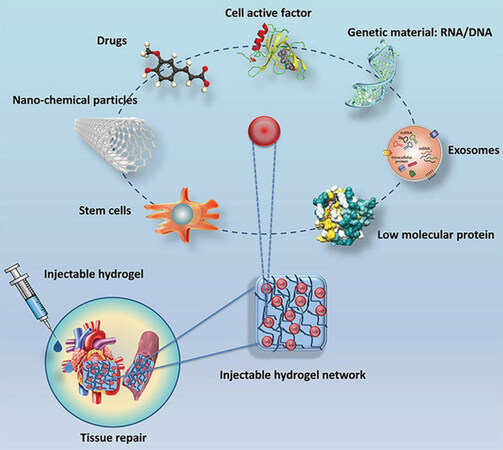
The cardiac ECM is made up of three major components, namely glycoproteins, proteoglycans, and glycosaminoglycans. Various glycoproteins such as fibronectin, laminin, fiber proteins, and prototypical matricellular proteins enrich the cardiac ECM. The major protein in cardiac ECM is fibrillar collagen. In mammalian hearts, cardiomyocyte proliferation may occur in neonates at a cardiac injury, but in adults, regeneration capacity is absent[49]. Cardiac ECM has a prominent effect in cardiac repair and regeneration, and changes vigorously after MI[50], yet the mechanical stiffness of free ECM is a major challenge in its use for PCI. Various approaches have been developed on the basis of ECM being used directly as the biomaterial for the cardiac tissue repair. ECM molecules are isolated and utilized directly as injectable hydrogels by intramyocardial injection or intracoronary perfusions. In these approaches, the injectable materials were supplemented with various materials such as DNA/RNA and cell active factors along with cardiac cells and growth factors as well, as shown in Figure 6, which synergistically helped in the repair of the cardiac tissues[51].
Figure 6. Representation of cardiac tissues using hydrogels, cardiac cells and growth factors. Adapted with permission from Liao et al.[51]
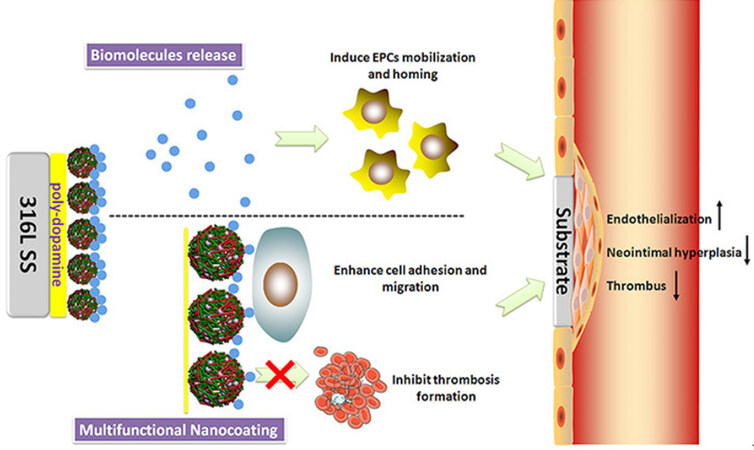
In a recent study, Du et al.[52] investigated the role of 5A/6A promoter polymorphism in the matrix metalloproteinase 3 (MMP-3) gene and in-stent restenosis (ISR). An increment in the ISRs with genotype proportion of 6A6A and a decrement in the 5A allele were reported. The findings suggested the role of gene-based cell proliferation in ISRs, hinting at the role of ECM interaction with the stents in cardiac tissue repair. Similarly, MMP-2 and -9 also play an important role in acute MI and cardiac tissue repair[53]. Hence, addition/delivery of these proteins may play a pivotal role in designing PCI with effective cardiac tissue repair. Growth factor impregnated nanomaterials play a vital role in CTE. In this direction, Mewhort et al.[54] proposed a surgical procedure using a CorMatrix-ECM biomaterial patch for the treatment of ischemic heart failure. The electrocardiography revealed an increment in the ejection fraction of basic fibroblasts, and prevention of left ventricle remodeling. The improvement in left ventricle contractility was confirmed by the pressure volume loop analysis. Various other factors such as coating of ECM and its biodegradation have also been tested for the improved healing/repair of the damaged cardiac tissue. In this regard, Liu et al.[55] performed the nanocoating of ECM-Inspired SDF-1α/Laminin for cardiac wound healing on the 316L stainless steel surface, as shown in Figure 7. It was found that the biomolecules were delivered in a controlled way at the site of action, and a promising approach was established to repair the cells after the injury. In addition, the designed layer inhibited platelet adhesion and activation, leading to controlled or reduced thrombosis and clot generation. The designed layer also enhanced endothelial cell migration and endothelial progenitor cell aggregation, resulting in faster cardiac tissue repair. All these studies showed that the incorporation of ECM and its constituents significantly affected the cellular repair and has a promising future if clubbed with PCI in the treatment of MI.
Figure 7. ECM-inspired nanocoating over stainless steel with enhanced wound repair. The modified surface effectively prevented thrombosis formation by inhibiting platelet adhesion and activation, while accelerating endothelial cell adhesion and migration. The controlled delivery of biomolecules induced the immobilization of EPC. Reprinted (adapted) with permission from Liu et al.[55]. Copyright (2017), American Chemical Society. EPCs: endothelial progenitor cells; ECM: extracellular matrix
Challenges and future perspectives
Various approaches have been applied in the treatment of MI using hydrogels with a varied degree of success. These approaches have been individually focusing on various factors such as mechanical strength, reduced thrombosis, tissue repair and cell regeneration. However, very few approaches have been attempted to incorporate all the desired properties in single stents to be used for PCI in the treatment of MI. Additionally, the hydrogels for PCI possessed major challenges in their mechanical strength, biodegradation, bioactivity and host body responses. Various hydrogel-based materials are being studied at the preclinical and clinical levels to be used for PCI. The selection of polymeric material is usually based on its cross-linking ability, interfacial interactions and enzymatic degradation[56]. These selections are usually based on the: (1) endogenous repair system of the host body leading to the challenges in mimicking of mechanical strength of the surrounding tissues; (2) the indigenous structure of polymers leading to the challenges in responses of cells and proteins; and (3) the salvage of the degraded polymeric debris leading to the challenges in enzymatic degradation. Hence, the major challenge remains to incorporate all these desired properties in a single stent, i.e., selection and/or design of a material with excellent bioactivity without compromising its mechanical strength and enzymatic degradation. In this direction, the chitosan- and alginate-based hydrogels have shown excellent properties in terms of their mechanical strength, biodegradability and cellular responses; however, their cytocompatibility solely depends on the degradation rate as well as degradation products. The monomers are usually non-toxic to cells[32], but the polymeric debris with specific orientation and their pharmacokinetic profiles affect the blood vessels and other body tissues as well[57]. Similarly, PEG-based materials have shown promising cell attachment and cardiac cell regeneration capacity but lack in biodegradation and also cytocompatibility. PEG is usually considered an antifouling agent[58], and doesn’t allow the non-specific adhesion of protein and cells. These properties can be utilized to coat it over stents for antifouling properties. ECM-based materials are considered the best tool for cardiac tissue repair; however, their poor mechanical strength limits their use alone for the synthesis of stents for PCI.
The drawbacks of these materials can be removed by blending them with each other or by creating a composite material of these fractions. In Section “ECM HYDROGELS IN CARDIAC TISSUE REPAIR”, it was seen that various growth factors and genes may provide excellent tissue repairability. Hence, nanomaterials based on chitosan, alginate and PEG can be impregnated with these ECM molecules. The delivery of these ECM materials can be tuned by controlling the molecular weight as well as the orientation of the composite structure. The cross-linking within the hydrogel and its respective mechanical strength and biodegradability can also be tuned. These approaches are advised to obtain site-specific materials for the designing of hydrogels for PCI. For example, the composite polymeric hydrogels can be designed to tune their mechanical strength as well as enzymatic degradation. One promising method is the construction of layer by layer structure for the stent material impregnated with ECM, which may provide the controlled release of ECM biomolecules and controlled biodegradation of the amalgamated materials. The composite material can also improve the enzyme-based degradation of the stents and reduce thrombosis. Furthermore, supplementation of cell cycle inhibitors, e.g., Rb1 and Meis2 and stem cells have been applied to engineer the cardiac tissue after MI. These approaches can be utilized to add biofunctionality to the hydrogels for the treatment of MI[59,60].
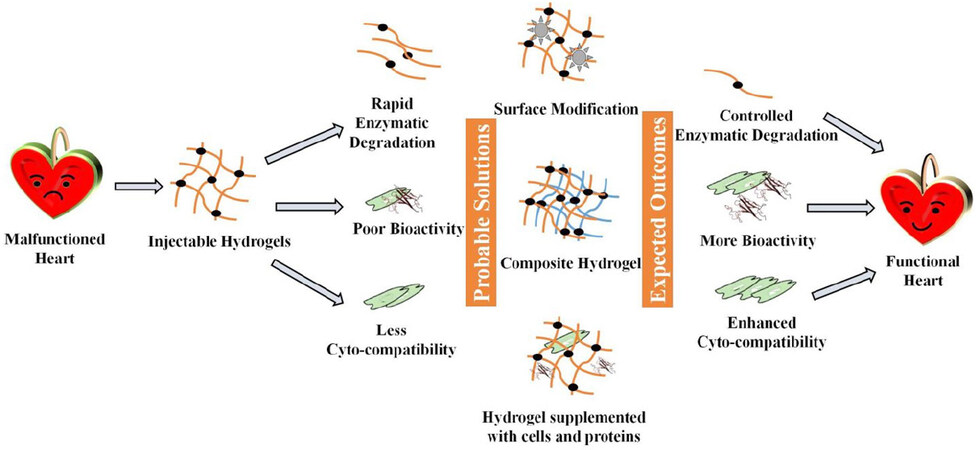
In addition, clot degrading agents such as heparin-based delivery system compiled with the stent hydrogels also seem to be an important tool in CTE[61]. Not much work has been done in this direction, but it can lead to a multifunctional stent material. Another drawback of hydrogel-based stent materials is their inability to kill bacteria, causing severe detrimental effects including immunogenic responses such as inflammation, arterial disruption, and hemorrhage[62]. Few of the materials have been modified with Ag NPs and drugs to enhance the antibacterial activity of chitosan and other polymers[9,10], but their application in stents has not been tested. Various peptide and peptoid materials have shown promising antibacterial activity[16,63,64] and can be incorporated in stent materials for PCI. Various surface-engineered drug delivery systems have been explored with excellent antibacterial and drug-releasing properties[65]. These approaches can be linked with hydrogels to bring the multi-functionality of drug release, mechanical stiffness and cardiac tissue repairing in stent materials. Target deficiency, i.e., the inability to be targeted at the site of action and biocompatibility are the major issues in biomaterial research. Recently, the effects of amine, octyl and mixed groups for surface modifications on protein attachment, orientation and cell adhesion have been scrutinized[66-68]. This strategy may be implemented to modify the surfaces of the stents for better protein interaction, biocompatibility and improved interfacial interactions as well. Interdisciplinary approaches may provide a better solution for cardiac tissue repair and reduce the harmful after-effects of MI. The major challenges of hydrogels, probable solutions and expected outcomes are depicted in Figure 8.
Conclusion
CTE is being extensively studied these days. Improper blood flow and damage to cardiac tissues are the major causes of MI. The blockages in blood vessels are the major factors that lead to MI. Various surgical and non-surgical studies have been performed to relieve the blockage in the coronary vessels, including PCI. Soft polymeric materials are constructed in the form of hydrogels, which are molded for making the stents to broaden the vessels for proper blood flow. Hydrogel-based materials have shown promising ability to be used for PCI; however, lack of the required mechanical strength, bioactivity and enzymatic degradation limits their practical applications. The controlled orientation of polymeric materials with specific bioactivity along with controlled enzymatic degradation has been major challenges for biomaterial researchers. There have been recent advances in the self-degrading hydrogel stents based on chitosan, alginate, PEG and various other polymeric materials. These materials have shown promising results at the laboratory scale to be utilized for PCI in the treatment of MI. Owing to their native properties, these materials have overcome many of the lags in PCI; however, they lack multi-functionality. Hence, interdisciplinary approaches to design a composite of these individual polymers blended with ECM biomolecules are proposed to develop promising materials for hydrogel-based stents in the treatment of MI.
Declarations
Authors’ contributionsPerformed data analysis and interpretation: Saxena V
Writing - original draft, review and editing: Saxena V, Pandey LM
Conceptualization, draft designing, formal analysis, supervision: Pandey LM
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2020.
REFERENCES
1. Roy A, Saxena V, Pandey LM. 3D printing for cardiovascular tissue engineering: a review. Mater Technol 2018;33:433-42.
2. Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852-66.
4. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, et al; SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72.
5. Wen Y, Li XY, Li ZY, Wang ML, Chen PP, et al. Intra-myocardial delivery of a novel thermosensitive hydrogel inhibits post-infarct heart failure after degradation in rat. J Cardiovasc Transl Res 2020; doi: 10.1007/s12265-019-09941-x.
6. Chen Y, Li C, Li C, Chen J, Li Y, et al. Tailorable hydrogel improves retention and cardioprotection of intramyocardial transplanted mesenchymal stem cells for the treatment of acute myocardial infarction in mice. J Am Heart Assoc 2020;9:e013784.
7. Cattelan G, Guerrero Gerbolés A, Foresti R, Pramstaller PP, Rossini A, et al. Alginate formulations: current developments in the race for hydrogel-based cardiac regeneration. Front Bioeng Biotechnol 2020;8:414.
8. Saxena V, Hasan A, Sharma S, Pandey LM. Edible oil nanoemulsion: an organic nanoantibiotic as a potential biomolecule delivery vehicle. Int J Polymeric Mater Polymeric Biomaterials 2017;67:410-9.
9. Hasan A, Waibhaw G, Tiwari S, Dharmalingam K, Shukla I, et al. Fabrication and characterization of chitosan, polyvinylpyrrolidone, and cellulose nanowhiskers nanocomposite films for wound healing drug delivery application. J Biomed Mater Res A 2017;105:2391-404.
10. Hasan A, Waibhaw G, Saxena V, Pandey LM. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int J Biol Macromol 2018;111:923-34.
11. Hasan A, Saxena V, Pandey LM. Surface functionalization of Ti6Al4V via self-assembled monolayers for improved protein adsorption and fibroblast adhesion. Langmuir 2018;34:3494-506.
12. Deka S, Saxena V, Hasan A, Chandra P, Pandey LM. Synthesis, characterization and in vitro analysis of α-Fe2O3-GdFeO3 biphasic materials as therapeutic agent for magnetic hyperthermia applications. Mater Sci Eng C Mater Biol Appl 2018;92:932-41.
13. Fopase R, Saxena V, Seal P, Borah J, Pandey LM. Yttrium iron garnet for hyperthermia applications: Synthesis, characterization and in-vitro analysis. Mater Sci Eng C 2020;116:111163.
14. Saxena V, Chandra P, Pandey LM. Design and characterization of novel Al-doped ZnO nanoassembly as an effective nanoantibiotic. Appl Nanosci 2018;8:1925-41.
15. Saxena V, Pandey LM. Bimetallic assembly of Fe(III) doped ZnO as an effective nanoantibiotic and its ROS independent antibacterial mechanism. J Trace Elem Med Biol 2020;57:126416.
16. Hasan A, Saxena V, Castelletto V, Zimbitas G, Seitsonen J, et al. Chain-end modifications and sequence arrangements of antimicrobial peptoids for mediating activity and nano-assembly. Front Chem 2020;8:416.
17. Saxena V, Pandey LM. Synthesis, characterization and antibacterial activity of aluminum doped zinc oxide. Mater Today Proc 2019;18:1388-400.
18. Si R, Gao C, Guo R, Lin C, Li J, et al. Human mesenchymal stem cells encapsulated-coacervated photoluminescent nanodots layered bioactive chitosan/collagen hydrogel matrices to indorse cardiac healing after acute myocardial infarction. J Photochem Photobiol B 2020;206:111789.
19. Uman S, Wang LL, Thorn SL, Liu Z, Duncan JS, et al. Imaging of injectable hydrogels delivered into myocardium with SPECT/CT. Adv Healthc Mater 2020;9:e2000294.
20. Navaee F, Renaud P, Braschler T. Highly efficient cardiac differentiation and maintenance by thrombin-coagulated fibrin hydrogels enriched with decellularized porcine heart extracellular matrix. bioRxiv 2020; doi: 10.1101/2020.01.30.927319.
21. Cheng J, Zhang P, Liu T, Zhang J. Preparation and properties of hydrogels based on PEG and isosorbide building blocks with phosphate linkages. Polymer 2015;78:212-8.
22. You F, Wu X, Kelly M, Chen X. Bioprinting and in vitro characterization of alginate dialdehyde-gelatin hydrogel bio-ink. Bio-des Manuf 2020;3:48-59.
23. Traverse JH, Henry TD, Dib N, Patel AN, Pepine C, et al. First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. J Am Coll Cardiol Basic Trans Sci 2019;4:659-69.
24. Curley CJ, Dolan EB, Otten M, Hinderer S, Duffy GP, et al. An injectable alginate/extra cellular matrix (ECM) hydrogel towards acellular treatment of heart failure. Drug Deliv Transl Res 2019;9:1-13.
25. Li T, Huang F, Diaz-Dussan D, Zhao J, Srinivas S, et al. Preparation and characterization of thermoresponsive PEG-based injectable hydrogels and their application for 3D cell culture. Biomacromolecules 2020;21:1254-63.
26. Obiweluozor FO, Tiwaria AP, Lee JH, Batgerel T, Kim JY, et al. Thromboresistant semi-IPN hydrogel coating: towards improvement of the hemocompatibility/biocompatibility of metallic stent implants. Mater Sci Eng C Mater Biol Appl 2019;99:1274-88.
27. Longchamp A, Kaur K, Macabrey D, Dubuis C, Corpataux J, et al. Hydrogen sulfide-releasing peptide hydrogel limits the development of intimal hyperplasia in human vein segments. Acta Biomater 2019;97:374-84.
28. Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, et al. Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischaemic injury. Nat Biomed Eng 2017;1:983-92.
29. Feng J, Wu Y, Chen W, Li J, Wang X, et al. Sustained release of bioactive IGF-1 from a silk fibroin microsphere-based injectable alginate hydrogel for the treatment of myocardial infarction. J Mater Chem B 2020;8:308-15.
30. Plotkin M, Vaibavi SR, Rufaihah AJ, Nithya V, Wang J, et al. The effect of matrix stiffness of injectable hydrogels on the preservation of cardiac function after a heart attack. Biomaterials 2014;35:1429-38.
31. Bahney CS, Lujan TJ, Hsu CW, Bottlang M, West JL, et al. Visible light photoinitiation of mesenchymal stem cell-laden bioresponsive hydrogels. Eur Cell Mater 2011;22:43-55. discussion 55
32. Islam S, Bhuiyan MAR, Islam MN. Chitin and chitosan: structure, properties and applications in biomedical engineering. J Polym Environ 2017;25:854-66.
33. Andrade F, Goycoolea F, Chiappetta DA, das Neves J, Sosnik A, et al. Chitosan-grafted copolymers and chitosan-ligand conjugates as matrices for pulmonary drug delivery. Int J Carbohydr Chem 2011;2011:865704.
34. Peng X, Yu Y, Wang Z, Zhang X, Wang J, et al. Potentiation effect of HB-EGF on facilitating wound healing via 2-N,6-O-sulfated chitosan nanoparticles modified PLGA scaffold. RSC Adv 2017;7:43161-71.
35. Qiu T, Jiang W, Yan P, Jiao L, Wang X. Development of 3D-printed sulfated chitosan modified bioresorbable stents for coronary artery disease. Front Bioeng Biotechnol 2020;8:462.
36. Lin M, Lou C, Lin J, Lin TA, Chou S, et al. Using spray-coating method to form PVA coronary artery stents: structure and property evaluations. J Polym Res 2018;25.
37. Khashi M, Hassanajili S, Golestaneh SI. Electrospun poly-lactic acid/chitosan nanofibers loaded with paclitaxel for coating of a prototype polymeric stent. Fibers Polym 2018;19:1444-53.
38. Hecht H, Srebnik S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016;17:2160-7.
39. Ravi, Pandey LM. Enhanced adsorption capacity of designed bentonite and alginate beads for the effective removal of methylene blue. Appl Clay Sci 2019;169:102-11.
40. Draget KI, Taylor C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocolloids 2011;25:251-6.
41. Sack KL, Aliotta E, Choy JS, Ennis DB, Davies NH, et al. Intra-myocardial alginate hydrogel injection acts as a left ventricular mid-wall constraint in swine. Acta Biomater 2020;111:170-80.
42. Qi Z, Liu X, Bai Y, Ge J. Alginate oligosaccharide inhibits platelet activation with minimal impact on bleeding time. Cardiol Plus 2020;5:42.
43. Lee CC, Su YC, Ko TP, Lin LL, Yang CY, et al. Structural basis of polyethylene glycol recognition by antibody. J Biomed Sci 2020;27:12.
44. Oesterhelt F, Rief M, Gaub HE. Single molecule force spectroscopy by AFM indicates helical structure of poly(ethylene-glycol) in water. New J Phys 1999;1:6.
45. Boyacioglu S, Kodal M, Ozkoc G. A comprehensive study on shape memory behavior of PEG plasticized PLA/TPU bio-blends. Eur Polym J 2020;122:109372.
46. Lin M, Lin J, Huang C, Chen Y. Textile fabricated biodegradable composite stents with core-shell structure. Polym Test 2020;81:106166.
47. Ge W, Zheng G, Ji X, He F, Hu J, et al. Effects of polyethylene glycol-20k on coronary perfusion pressure and postresuscitation myocardial and cerebral function in a rat model of cardiac arrest. J Am Heart Assoc 2020;9:e014232.
48. Aykar SS, Reynolds DE, Mcnamara MC, Hashemi NN. Manufacturing of poly(ethylene glycol diacrylate)-based hollow microvessels using microfluidics. RSC Adv 2020;10:4095-102.
49. Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, et al. Transient regenerative potential of the neonatal mouse heart. Science 2011;331:1078-80.
50. Li H, Bao M, Nie Y. Extracellular matrix-based biomaterials for cardiac regeneration and repair. Heart Fail Rev 2020; doi: 10.1007/s10741-020-09953-9.
51. Liao X, Yang X, Deng H, Hao Y, Mao L, et al. Injectable hydrogel-based nanocomposites for cardiovascular diseases. Front Bioeng Biotechnol 2020;8:251.
52. Du JB, Zhang W, Li N, Jiang H, Liu Y, et al. Association study of matrix metalloproteinase 3 5A/6A polymorphism with in-stent restenosis after percutaneous coronary interventions in a Han Chinese population. J Int Med Res 2020;48:300060519827145.
53. Owolabi US, Amraotkar AR, Coulter AR, Singam NSV, Aladili BN, et al. Change in matrix metalloproteinase 2, 3, and 9 levels at the time of and after acute atherothrombotic myocardial infarction. J Thromb Thrombolysis 2020;49:235-44.
54. Mewhort HE, Turnbull JD, Meijndert HC, Ngu JM, Fedak PW. Epicardial infarct repair with basic fibroblast growth factor-enhanced CorMatrix-ECM biomaterial attenuates postischemic cardiac remodeling. J Thorac Cardiovasc Surg 2014;147:1650-9.
55. Liu T, Wang X, Tang X, Gong T, Ye W, et al. Surface modification with ECM-inspired SDF-1α/laminin-loaded nanocoating for vascular wound healing. ACS Appl Mater Interfaces 2017;9:30373-86.
56. Hasan A, Pandey LM. Review: polymers, surface-modified polymers, and self assembled monolayers as surface-modifying agents for biomaterials. Polymer-Plastics Technol Eng 2015;54:1358-78.
57. Igarashi E. Factors affecting toxicity and efficacy of polymeric nanomedicines. Toxicol Appl Pharmacol 2008;229:121-34.
58. Zhang S, Cao J, Ma N, You M, Wang X, et al. Fast and facile fabrication of antifouling and hemocompatible PVDF membrane tethered with amino-acid modified PEG film. Appl Surf Sci 2018;428:41-53.
59. Alam P, Haile B, Arif M, Pandey R, Rokvic M, et al. Inhibition of senescence-associated genes Rb1 and Meis2 in adult cardiomyocytes results in cell cycle reentry and cardiac repair post–myocardial infarction. J Am Heart Assoc 2019;8:e012089.
60. Waters R, Alam P, Pacelli S, Chakravarti AR, Ahmed RPH, et al. Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater 2018;69:95-106.
61. Plautz WE, Sekhar Pilli VS, Cooley BC, Chattopadhyay R, Westmark PR, et al. Anticoagulant protein S targets the factor IXa heparin-binding exosite to prevent thrombosis. Arterioscler Thromb Vasc Biol 2018;38:816-28.
62. Bosman WM, Borger van der Burg BL, Schuttevaer HM, Thoma S, Hedeman Joosten PP. Infections of intravascular bare metal stents: a case report and review of literature. Eur J Vasc Endovasc Surg 2014;47:87-99.
63. Hasan A, Lee K, Tewari K, Pandey LM, Messersmith PB, et al. Surface design for immobilization of an antimicrobial peptide mimic for efficient anti-biofouling. Chemistry 2020;26:5789-93.
64. Saxena V, Merrilees MG, Lau KHA. Antifouling peptoid biointerfaces. In: Chandra P, Pandey LM, editors. Biointerface engineering: prospects in medical diagnostics and drug delivery. Springer; 2020. pp. 55-73.
65. Fopase R, Bhardwaj A, Yadav VS, Pandey LM. Engineered drug delivery systems: insights of biointerface. In: Chandra P, Pandey LM, editors. Biointerface engineering: prospects in medical diagnostics and drug delivery. Springer; 2020. pp. 1-30.
66. Pandey LM, Le Denmat S, Delabouglise D, Bruckert F, Pattanayek SK, et al. Surface chemistry at the nanometer scale influences insulin aggregation. Colloids Surf B Biointerfaces 2012;100:69-76.
67. Hasan A, Pandey LM. Kinetic studies of attachment and re-orientation of octyltriethoxysilane for formation of self-assembled monolayer on a silica substrate. Mater Sci Eng C Mater Biol Appl 2016;68:423-9.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Saxena V, Pandey LM. Nanomaterial-based hydrogels for coronary interventions: a mini review. Mini-invasive Surg 2020;4:62. http://dx.doi.org/10.20517/2574-1225.2020.68
AMA Style
Saxena V, Pandey LM. Nanomaterial-based hydrogels for coronary interventions: a mini review. Mini-invasive Surgery. 2020; 4: 62. http://dx.doi.org/10.20517/2574-1225.2020.68
Chicago/Turabian Style
Saxena, Varun, Lalit M. Pandey. 2020. "Nanomaterial-based hydrogels for coronary interventions: a mini review" Mini-invasive Surgery. 4: 62. http://dx.doi.org/10.20517/2574-1225.2020.68
ACS Style
Saxena, V.; Pandey LM. Nanomaterial-based hydrogels for coronary interventions: a mini review. Mini-invasive. Surg. 2020, 4, 62. http://dx.doi.org/10.20517/2574-1225.2020.68
About This Article
Copyright
Data & Comments
Data
 Cite This Article 9 clicks
Cite This Article 9 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.