The prognostic impact of frailty in patients undergoing percutaneous mitral valve repair
Abstract
Aim: Percutaneous mitral valve repair (PMVR) with MitraClip® has proven to be an effective therapy to reduce mitral regurgitation in patients at high risk for conventional surgery. This population is currently characterized by advance age and high prevalence of comorbidities. Our aim was to evaluate the prevalence of frailty in a cohort of patients undergoing PMVR and its impact on clinical outcomes during follow-up.
Methods: A prospective registry was performed including all consecutive patients who underwent elective PMVR between June 2014 and March 2018 in our institution. Frailty was evaluated at admission with the functional FRAIL scale. In-hospital and 30-day procedural outcomes were collected. Clinical follow up was carried out including New York Heart Association (NYHA) functional class, heart failure hospitalization and death.
Results: Overall, 70 patients were included (mean age 75.3 ± 9.9 years, 65.7% male). Among them, 27 patients (38.6%) had a pre-procedural FRAIL score greater than 2, meeting frailty criteria. No differences between frail and non-frail patients were found in technical success (P = 1.0) or 30-day device success (P = 0.739). At six months follow up, both groups showed a significant improvement in NYHA functional class compared to baseline (frail: P = 0.002; non-frail: P < 0.001). During a median follow up of 675 (range 416-976) days, frailty patients had a higher incidence of HF admission and all-cause mortality (P = 0.013). In multivariate COX regression analysis, FRAIL score greater than 2 was significantly related to the primary composite endpoint (HR = 2.45; 95%CI: 1.02-5.88; P = 0.044).
Conclusion: Frailty was common in patients undergoing PMVR in our institution. Despite post-procedural clinical improvement, frailty was related to adverse outcomes in our series.
Keywords
Introduction
In the last decade, percutaneous mitral valve repair (PMVR) with MitraClip® device (Abbot Vascular, Santa Clara, USA) has proven to be an effective therapy to reduce mitral regurgitation (MR), with a low incidence of complications in patients deemed as high-risk candidates or unfit for conventional surgery[1]. Risk stratification in these patients is challenging and it is usually based on non-dedicated risk scores developed in the surgery field, with a modest predictive value in this scenario[2].
Frailty is a clinical syndrome related to aging and characterised by a decrease in so-called “biological reserve” against a stressful event, which implies a situation of vulnerability in the case of intercurrent disease or medical issues requiring hospitalization[3]. Current candidates for PMVR are characterized by advanced age and a high prevalence of cardiovascular and non-cardiovascular comorbidities[4,5]. All these factors have been commonly related to frailty. The main objective of our study was to assess the prevalence of frailty in a cohort of patients undergoing PMVR, and to assess its impact on clinical outcomes during follow-up.
Methods
Study population
A prospective registry was performed including of all consecutive patients with symptomatic MR grade 3+ or 4+ who underwent elective PMVR in our center between June 2014 and March 2018. Those who received a MitraClip® as an urgent procedure (n = 15), during an admission for decompensated heart failure (HF), were excluded from the analysis.
Study procedures
The indications for PMVR in each patient were discussed by an interdisciplinary Heart Team, including clinical and interventional cardiologists, cardiac surgeons, and imaging specialists. Pre-procedural transthoracic and transesophageal echocardiography was performed in all patients to assess the severity of MR and the anatomical suitability for clip implantation, following current recommendations from the European Association of Cardiovascular Imaging for valvular heart disease assessment[6]. PMVR was carried out under general anesthesia with guidance of fluoroscopy and transesophageal echocardiography.
Frailty was assessed according to functional FRAIL scale at admission for elective PMVR[7]. The FRAIL questionnaire includes 5 components (Fatigue, Resistance, Ambulation, Illness and Loss of weight) and scores range from 0 to 5, considering as frail patients with score of 3-5. Baseline characteristics, echocardiographic and biochemical findings were collected. Procedural and 30-day clinical and echocardiographic outcomes were collected. New York Heart Association (NYHA) functional class was documented at the 6-month scheduled outpatient clinic after discharge. Long-term clinical follow-up was performed including primary re-admission for heart failure (HF) and death from any cause.
Study outcomes
Procedural results and adverse outcomes during follow-up were defined according to the “Mitral Valve Academic Research Consortium”[8]. Technical success was defined as the implantation of at least 1 clip in the absence of procedural mortality or the need for emergency cardiovascular surgery. Device success at 30 days was defined as the implantation of at least 1 clip with residual MR ≤ 2+ and transmitral valvular mean gradient < 5 mmHg, in the absence of major adverse events (death, stroke, unscheduled cardiovascular intervention, or device detachment). Device-related complications such as fracture, migration, embolization or partial detachment were considered as structural device failure. Functional failure was defined as the suboptimal result of PMVR during follow up (residual or recurrent MR 3+ or 4+ and/or transmitral mean gradient ≥ 5 mmHg). Anemia was defined according to the World Health Organization as a concentration of serum hemoglobin < 12 g/dL in women and < 13 g/dL in men[9]. A composite primary endpoint of readmission for HF and all-cause death was established to define the prognostic impact of frailty in this series.
Statistical analysis
Continuous variables were summarized as mean ± standard deviation or as medians and interquartile range, and were compared using unpaired Student t-tests or the non-parametric Wilcoxon rank sum tests if the normal distribution of the variables could not be demonstrated. Derangement from the normal distribution was assessed with the Shapiro-Wilk test. Categorical variables were described as percentages and compared using Chi-square or Fisher exact tests accordingly to expected frequency over or below 5, respectively. Survival curves for time-to-event were constructed on the basis of all available follow-up data using Kaplan-Meier estimates, and comparisons between frail and non-frail PMVR patients were performed using the log-rank test. Cox regression multivariate analysis was performed to evaluate the prognostic impact of frailty as an independent predictor for HF hospitalizations and all-cause mortality. Variables found to be statistically significant in the univariate analysis as well as others with clinical interest were included as covariates in the multivariable model. A P-value < 0.05 was regarded as statistically significant. Statistical analyses were performed using STATA software version 14.2.
Results
In the study period, 70 patients (age 75.3 ± 9.9 years, 65.7% male) underwent elective PMVR in our center.
Study population
Baseline characteristics of patients included in the study cohort are summarized in Table 1, grouped by the presence of frailty criteria. Almost all patients (94.3%) had been admitted previously for HF, or were in advanced NYHA functional class III or IV. The etiology of the MR was predominantly functional, and patients were considered to be at high risk for conventional surgery according to surgical risk scales or Heart Team consensus. The prevalence of comorbidities was similar to other contemporary cohorts.
Baseline characteristics of patients included in the study cohort
| All patients (n = 70) | Frail (n = 27) | Non frail (n = 43) | P value | |
|---|---|---|---|---|
| Age (years) | 75.3 ± 9.9 | 77.8 ± 9.0 | 73.7 ± 10.2 | 0.043 |
| Male (%) | 65.7 | 66.7 | 65.1 | 0.894 |
| Body mass index (kg/m2) | 27.2 ± 5.5 | 25.6 ± 4.3 | 28.2 ± 6.0 | 0.030 |
| Serum albumin < 4 g/dL (%) | 44.3 | 59.3 | 34.9 | 0.046 |
| Smoking (%) | 40.0 | 44.4 | 37.2 | 0.548 |
| Hypertension (%) | 65.7 | 77.8 | 58.1 | 0.092 |
| Diabetes (%) | 27.1 | 25.9 | 27.9 | 0.856 |
| Chronic obstructive pulmonary disease (%) | 30.0 | 40.7 | 23.3 | 0.120 |
| Glomerular filtrate rate ≤ 60 mL/min (%) | 47.1 | 59.3 | 39.5 | 0.108 |
| Peripheral arteriopathy (%) | 22.9 | 29.6 | 18.6 | 0.285 |
| Stroke (%) | 14.3 | 18.5 | 11.6 | 0.493 |
| Cognitive impairment (%) | 7.1 | 14.8 | 2.3 | 0.069 |
| Anemia (%) | 44.3 | 59.3 | 34.9 | 0.046 |
| Ischemic cardiopathy (%)
Acute myocardial infarction Coronary revascularization Percutaneous coronary intervention Coronary artery bypass grafting | 48.6
31.4 40.0 34.3 14.3 | 48.2
37.0 40.7 33.3 18.5 | 48.8
27.9 39.5 34.9 11.6 | 0.955
0.423 0.920 0.894 0.423 |
| Non-coronary cardiac surgery (%) | 14.3 | 11.1 | 16.3 | 0.730 |
| Implantable cardiac device (%) | 31.4 | 22.2 | 37.2 | 0.189 |
| Atrial fibrillation (%) | 62.9 | 66.7 | 60.5 | 0.601 |
| Left ventricular ejection fraction (%) | 40.5 ± 16.3 | 41.1 ± 16.9 | 40.1 ± 16.1 | 0.597 |
| Mitral regurgitation (%)
3+ 4+ | 10.0
90.0 | 11.1
88.9 | 9.3
90.7 | 1.0 |
| Etiology of mitral regurgitation (%)
Degenerative o mixed Ischemic functional Non-ischemic | 27.1
35.7 37.1 | 33.3
40.7 25.9 | 23.3
32.6 44.2 | 0.298 |
| Severe pulmonary hypertension (%) | 22.9 | 29.6 | 18.6 | 0.285 |
| Prior heart failure admission within 12 months | 1 [1-2] | 2 [1-3] | 1 [1-2] | 0.044 |
| NYHA functional class (%)
II III IV | 18.6
67.1 14.3 | 14.8
59.3 25.9 | 20.9
72.1 7.0 | 0.104 |
| NT-pro brain natriuretic peptide (pg/mL) | 2030.5 [1126.0-3428.0] | 2962.0 [1525.0-9059.0] | 2030.5 [1126.0-3428.0] | 0.070 |
| Euro score logistic (%) | 17.4 [10.1-30.3] | 21.6 [11.1-36.9] | 16.0 [9.2-29.2] | 0.151 |
| Society of thoracic surgeons (%) | 3.6 [1.9-5.4] | 4.2 [2.4-6.1] | 2.8 [1.2-5.4] | 0.060 |
| Seattle heart failure risk score (%) | 16.6 [10.5-20.3] | 19.8 [14.0-30.6] | 15.4 [8.4-19.5] | 0.005 |
FRAIL questionnaire scores showed the following distribution in the cohort: score 0, 5.71%; score 1, 22.9%; score 2, 32.9%; score 3, 28.6%; score 4, 10.0%; score 5, 0%. Overall, 27 patients (38.6%) had a FRAIL score greater than 2, meeting frailty criteria. Patients classified as frail were older (P = 0.043), and had lower body mass index (0.030), higher prevalence of low serum albumin < 4 g/dL (P = 0.046), and anemia (0.046), higher number of admissions for HF in the previous year (P = 0.044), and worse prognosis estimated by Seattle HF risk score (P = 0.005). Likewise, they presented worse pre-procedural NYHA functional class (P = 0.104), higher levels of NT-proBNP (P = 0.070), and higher surgical risk (P = 0.060), as well as a higher prevalence of comorbidities (hypertension, advanced kidney disease, chronic obstructive pulmonary disease, or cognitive impairment), although this did not reach statistical significance.
Procedural results
At least one clip was successfully implanted in all patients, and 28 cases (40%) were treated with 2 or more clips. No significant differences were found in the duration of the procedure (P = 0.749), the time of fluoroscopy (P = 0.768), or the number of clips implanted between frail and non-frail patients (P = 0.359, Table 2). One patient (1.4%) underwent emergency valve replacement due to rupture of the subvalvular apparatus with massive residual MR after MitraClip® deployment. Overall, procedural technical success was 98.6%, with no differences between the two groups (P = 1.0). Likewise, no significant differences in the incidence of major procedural complications were observed during hospitalization.
Short-term procedural outcomes
| All patients (n = 70) | Frail (n = 27) | Non frail (n = 43) | P value | ||
|---|---|---|---|---|---|
| Days of admission | 4 [4-5] | 4 [4-5] | 4 [4-5] | 0.625 | |
| Procedure | Device time (min) | 62.5 [45.0-86.0] | 70.0 [42.0-95.0] | 60.0 [45.0-85.0] | 0.749 |
| Fluoroscopy time (min) | 40.8 [34.3-50.0] | 43.4 [34.4-54.0] | 40.4 [34.1-50.0] | 0.768 | |
| Number of clips | 1 [1-2] | 1 [1-2] | 1 [1-2] | 0.359 | |
| Emergent cardiac surgery (%) | 1.4 | 0 | 2.3 | 1.0 | |
| Procedural death (%) | 0 | 0 | 0 | 1.0 | |
| Technical success (%) | 98.6 | 100 | 97.7 | 1.0 | |
| 30-day | Cardiovascular intervention (%) | 4.3 | 0 | 7.0 | 0.279 |
| Stroke (%)
Transient ischemic attack (%) | 0
2.9 | 0
3.7 | 0
2.3 | 1.0 | |
| Major vascular complication (%) | 2.9 | 3.7 | 2.3 | 1.0 | |
| Major structural complication (%) | 0 | 0 | 0 | 1.0 | |
| Major bleeding (%) | 7.1 | 11.1 | 4.7 | 0.367 | |
| Life-threatening or fatal bleeding (%) | 0 | 0 | 0 | 1.0 | |
| Acute kidney injury grade 2 or 3 (%) | 0 | 0 | 0 | 1.0 | |
| Death (%) | 1.4 | 3.7 | 0 | 0.386 | |
| Structural failure (%) | 1.4 | 3.7 | 0 | 0.386 | |
| Functional failure (%) | 8.6 | 11.1 | 7.0 | 0.670 | |
| Device success (%) | 84.3 | 81.5 | 86.1 | 0.739 | |
At 30-day echocardiographic follow-up, one patient (1.4%) had partial clip detachment with severe residual MR, and 6 patients (8.6%) had residual MR > 2+ and/or transmitral mean gradient ≥ 5 mmHg. One patient with very severe left ventricular dysfunction died within 30 days after discharge. Overall, device success rate at 30 days was 84.3%, with no differences between frail and non-frail groups (P = 0.739).
Clinical outcomes
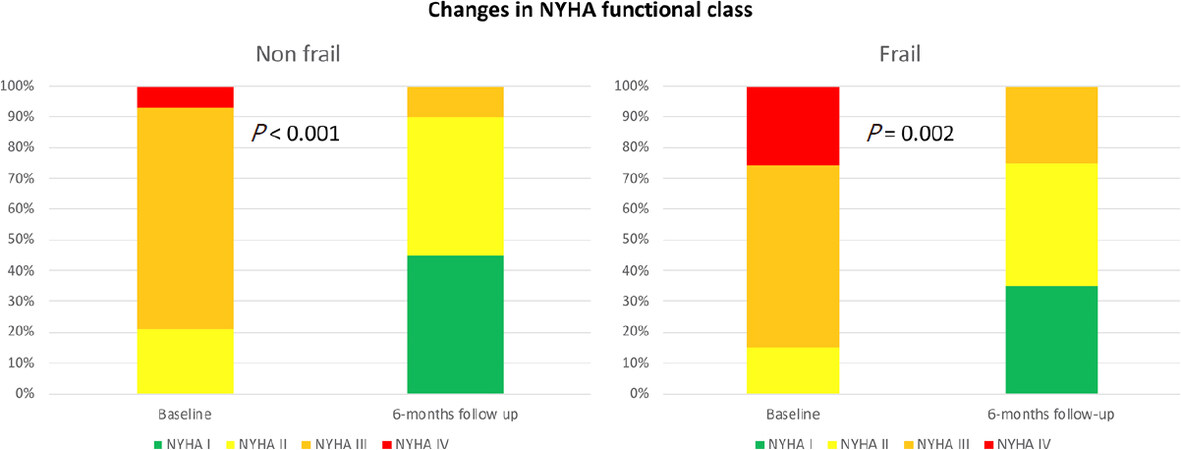
At 6-month follow up, both groups of patients showed a significant improvement in NYHA functional class (frail: P = 0.002; non-frail: P < 0.001; Figure 1). During a median follow-up of 675 days (range 416-976), 22 patients (31.4%) were hospitalized due to HF and 16 patients (22.9%) died. The composite endpoint of readmission for HF or death from any cause occurred in 30 patients (42.9%).
Figure 1. Changes in NYHA functional class according to frailty status. NYHA: New York Heart Association
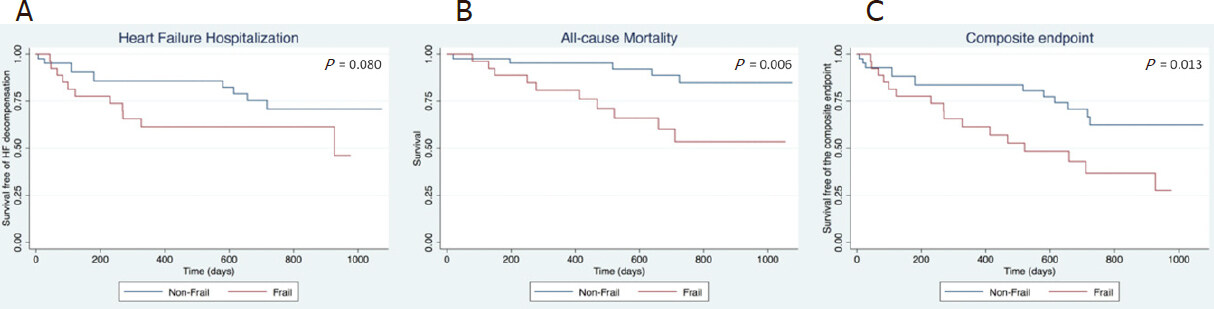
Frail patients had a non-significantly higher rate of HF hospitalization (log-rank test: P = 0.080, Figure 2A), and a lower survival (log-rank test: P = 0.006, Figure 2B), compared to non-frail patients. Survival free of the composite endpoint was significantly lower in the group of frail patients (log-rank test: P = 0.013, Figure 2C), with a probability of survival with no HF re-admission at one-year follow up of 61.6% vs. 83.7% in frail and non-frail patients, respectively.
Figure 2. Kaplan-Meier curves displaying survival free from heart failure or death. A: Heart failure rehospitalizations. Frail patients experienced higher prevalence during follow-up; B: All cause mortality. Frail patients showed higher death rate than non frail patients; C: Composite end-point (death/readmission due to heart failure). Frail patients showed worse outcome.
In Cox regression analysis, frailty was significantly related to the composite endpoint in the univariate analysis (HR = 2.59; 95%CI: 1.24-5.41; P = 0.011). This association was not significantly modified (HR = 2.45; 95%CI: 1.02-5.88; P = 0.044) after adjusting in a multivariate model including the following variables: age, diabetes mellitus, advanced chronic kidney disease (stage IIIb-V), pre-procedural NYHA functional class, high Seattle HF risk score, and atrial fibrillation.
Discussion
This study analyzed the prevalence of frailty in a single-center cohort of patients undergoing elective PMVR, and its impact on clinical outcomes during a median follow-up over 1 year. The main findings of our report were the following: (1) the prevalence of frailty in this series was high (about 2 out of 5 patients); (2) no differences in procedural outcomes and short-term device success rates were observed between frail and non-frail patients; (3) NYHA functional class significantly improved in both groups at 6-months follow-up after PMVR; and (4) frailty was significantly related to a higher risk of HF readmission or death from any cause during long-term follow-up.
Prevalence of frailty among patients with cardiovascular disease ranges between 25% to 50%, depending on the scales used and the clinical setting[10]. In addition, many reports have shown a higher incidence of adverse events in frailty patients with ischemic heart disease, HF, or those undergoing cardiac surgery, or percutaneous intervention for either coronary or structural heart disease[11-14]. In the latter scenario, several studies have pointed out that patients deemed as frail who undergo percutaneous aortic valve replacement have worse prognosis than those that do not meet frailty criteria[14,15]. Similar findings have been reported in patients undergoing PMVR. In this regard, Metze et al.[16] observed a prevalence of frailty according to the FRIED score of 45.5% in a cohort of more than 200 patients who received MitraClip®. In this series, device success rates were similar among frail and non-frail patients, and a significant improvement was observed in both groups in the NYHA functional class, 6-min walk test, and quality of life questionnaires. However, frailty was significantly related to a higher probability of readmission for HF or death from any cause during a median follow-up of more than 1 year. Likewise, in our study, the presence of frailty according to the FRAIL score was associated with a more than two-fold increase in the incidence of the composite endpoint, despite similar short-term procedural results and functional improvement.
Multiple frailty scales have been validated in different clinical settings[3]. Some, such as the FRIED score, focus on physical strength and walking speed. This “uni-dimensional” approach has a higher predictive value in some scenarios of cardiovascular disease, although their use in daily practice is limited by its greater complexity and time demands[17]. On the other hand, the “multidimensional” approach, including the FRAIL scale, considers that frailty is an accumulation of comorbidities, deficits and symptoms involving one or more domains of human functioning. These scores are based on clinical questionnaires and the subjective judgment of the healthcare provider. The advantages of this approach are that it is simple to perform and can be used in patients with any stage of disability as a screening test. To the best of our knowledge, this is the first study to evaluate the prognosis impact of the FRAIL score in PMVR.
In between both scores, a modified FRAIL scale has been recently suggested, adding a rapid physical test (e.g., the ability to get up from the chair), a questionnaire to address cognitive impairment, and two laboratory parameters (serum albumin and hemoglobin) to the traditional score[14]. This “Essential Frailty Toolskit” demonstrated a greater predictive value for adverse events compared to other scales in patients with severe aortic stenosis undergoing either surgical or percutaneous valve replacement. Further studies are needed to assess its usefulness in patients undergoing other structural interventional procedures.
PMVR with MitraClip® has proven to persistently reduce MR with low rates of procedural complications in patients at high surgical risk[18]. Furthermore, observational registries have shown a significant improvement in functional class, 6-minute walk test and quality of life[19]. More recently, data from randomized controlled trials suggest that there might be a survival benefit of MitraClip® compared to stand-alone medical therapy in patients with functional MR[20-22]. Nevertheless, selection of patients in order to find those who will benefit the most from PMVR and avoid futility is still extremely challenging. In this regard, pre-procedural evaluation of frailty might help to identify those patients with very poor short-term prognosis and those at a higher risk of non-cardiovascular mortality[23]. Although there is extensive evidence of the prognosis impact of frailty in cardiovascular disease, some aspects should be taken into account. First, frailty, in the absence of advanced disability, is a potentially reversible and treatable condition, so that a pre-procedural intervention could hypothetically improve the clinical prognosis of patients at high risk[24]. Second, the latest recommendations of the geriatric societies do not consider frailty as a contraindication to any invasive treatment but, on the contrary, as an important assessment element to establish an individualized plan of care[3]. Therefore, frailty should be an additive point to address by the multidisciplinary Heart Team when considering a potential candidate for MitraClip®, never a single tool for decision making. Risk stratification of patients undergoing PMVR is currently based on non-dedicated scales developed in the surgical field with a modest power of discrimination in this scenario[2]. The implementation of frailty scales might improve selection of patients[25]. Finally, despite an worse prognosis, frail patients showed a significant clinical improvement in the short-term and, therefore, this therapy might be considered for symptomatic relief in the absence of other reliable alternatives, even in the absence of consistent survival benefit.
Limitations
This study has some limitations. First of all, it is a single center small cohort of patients. Second, no dedicated test has been included to assess physical frailty, such as pressure force or gait speed. Third, frailty was not re-evaluated during follow up.
In conclusion, frailty was a frequent finding among patients undergoing PMVR. The presence of this syndrome did not impact procedural success. Despite symptomatic improvement in this patient group after PMVR, frailty was associated with an increase in adverse outcomes during follow-up. Further studies are needed to validate our results, and to assess whether any intervention to improve this syndrome can modify the prognosis of this patient group.
Declarations
Authors’ contributionsMade substantial contributions to conception and design of the study and performed data analysis and interpretation: Benito-González T, Estévez-Loureiro R
Performed data acquisition, as well as provided administrative, technical, and material support: del Castillo S, Minguito-Carazo C, Echarte-Morales J, Garrote-Coloma C
Drafted the paper: Benito-González T, Estévez-Loureiro R, Garrote-Coloma C
Reviewed critically: Fernández-Vázquez F
Gave final approval: Benito-González T, Estévez-Loureiro R, del Castillo S, Minguito-Carazo C, Echarte-Morales J, Garrote-Coloma C, Fernández-Vázquez F
Availability of data and materialsData of this work can be provided in case of formal request and under a legitimate cause.
Financial support and sponsorshipNone.
Conflicts of interestBenito-González T received an unrestricted research grant from Abbot Vascular not related to this report; Estévez-Loureiro R and Garrote-Coloma C are proctors for MitraClip®.
Ethical approval and consent to participateThis research has been conducted in accordance with the Declaration of Helsinki and approved by University Hospital of Leon ethics committee. All participants gave informed consent.
Consent for publicationNot applicable.
Copyright© The Author(s) 2020.
REFERENCES
1. Franzen O, Baldus S, Rudolph V, Meyer S, Knap M, et al. Acute outcomes of MitraClip therapy for mitral regurgitation in high-surgical-risk patients: emphasis on adverse valve morphology and severe left ventricular dysfunction. Eur Heart J 2010;31:1373-81.
2. Kortlandt FA, van ‘t Klooster CC, Bakker AL, Swaans MJ, Kelder JC, et al. The predictive value of conventional surgical risk scores for periprocedural mortality in percutaneous mitral valve repair. Neth Heart J 2016;24:475-80.
3. Díez-villanueva P, Arizá-solé A, Vidán MT, Bonanad C, Formiga F, et al. Recomendaciones de la Sección de Cardiología Geriátrica de la Sociedad Española de Cardiología para la valoración de la fragilidad en el anciano con cardiopatía. Rev Esp Cardiol 2019;72:63-71.
4. Zuern CS, Bauer A, Lubos E, Boekstegers P, Puls M, et al. Influence of non-cardiac comorbidities on outcome after percutaneous mitral valve repair: results from the German transcatheter mitral valve interventions (TRAMI) registry. Clin Res Cardiol 2015;104:1044-53.
5. Maisano F, Franzen O, Baldus S, Schäfer U, Hausleiter J, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol 2013;62:1052-61.
6. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, et al; Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2013;14:611-44.
7. Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc 2008;9:71-2.
8. Stone GW, Adams DH, Abraham WT, Kappetein AP, Généreux P, et al; Mitral Valve Academic Research Consortium (MVARC). Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: Part 2: endpoint definitions: a consensus document from the mitral valve academic research consortium. J Am Coll Cardiol 2015;66:308-21.
9. Wei CC, Zhang ST, Tan G, Zhang SH, Liu M. Impact of anemia on in-hospital complications after ischemic stroke. Eur J Neurol 2018;25:768-74.
10. Afilalo J. Frailty in patients with cardiovascular disease: why, when, and how to measure. Curr Cardiovasc Risk Rep 2011;5:467-72.
11. Sanchis J, Bonanad C, Ruiz V, Fernández J, García-Blas S, et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J 2014;168:784-91.
12. Uchmanowicz I, Łoboz-Rudnicka M, Szeląg P, Jankowska-Polańska B, Łoboz-Grudzień K. Frailty in heart failure. Curr Heart Fail Rep 2014;11:266-73.
13. Yanagawa B, Graham MM, Afilalo J, Hassan A, Arora RC. Frailty as a risk predictor in cardiac surgery: Beyond the eyeball test. J Thorac Cardiovasc Surg 2019;157:1905-9.
14. Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol 2017;70:689-700.
15. Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv 2012;5:974-81.
16. Metze C, Matzik AS, Scherner M, Körber MI, Michels G, et al. Impact of frailty on outcomes in patients undergoing percutaneous mitral valve repair. JACC Cardiovasc Interv 2017;10:1920-9.
17. White HD, Westerhout CM, Alexander KP, Roe MT, Winters KJ, et al; TRILOGY ACS investigators. Frailty is associated with worse outcomes in non-ST-segment elevation acute coronary syndromes: insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Eur Heart J Acute Cardiovasc Care 2016;5:231-42.
18. Benito-gonzález T, Estévez-loureiro R, Cardona JG, Prado APD, Ruiz MC, et al. Percutaneous treatment of mitral and tricuspid regurgitation in heart failure. In: Akin I, editor. Interventional Cardiology. InTech; 2017.
19. Iliadis C, Lee S, Kuhr K, Metze C, Matzik AS, et al. Functional status and quality of life after transcatheter mitral valve repair: a prospective cohort study and systematic review. Clin Res Cardiol 2017;106:1005-17.
20. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, et al; COAPT Investigators. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307-18.
21. Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, et al; MITRA-FR Investigators. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297-306.
22. Benito-González T, Estévez-Loureiro R, Villablanca PA, Armeni P, Iglesias-Gárriz I, et al. Percutaneous mitral valve repair vs. stand-alone medical therapy in patients with functional mitral regurgitation and heart failure. Cardiovasc Revasc Med 2020;21:52-60.
23. Moretti C, Iqbal J, Murray S, Bertaina M, Parviz Y, et al. Prospective assessment of a palliative care tool to predict one-year mortality in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2017;6:272-9.
24. Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2017;9:CD006211.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Benito-González T, Estévez-Loureiro R, del Castillo S, Minguito-Carazo C, Echarte-Morales J, Garrote-Coloma C, Fernández-Vázquez F. The prognostic impact of frailty in patients undergoing percutaneous mitral valve repair. Mini-invasive Surg 2020;4:67. http://dx.doi.org/10.20517/2574-1225.2020.54
AMA Style
Benito-González T, Estévez-Loureiro R, del Castillo S, Minguito-Carazo C, Echarte-Morales J, Garrote-Coloma C, Fernández-Vázquez F. The prognostic impact of frailty in patients undergoing percutaneous mitral valve repair. Mini-invasive Surgery. 2020; 4: 67. http://dx.doi.org/10.20517/2574-1225.2020.54
Chicago/Turabian Style
Benito-González, Tomás, Rodrigo Estévez-Loureiro, Samuel del Castillo, Carlos Minguito-Carazo, Julio Echarte-Morales, Carmen Garrote-Coloma, Felipe Fernández-Vázquez. 2020. "The prognostic impact of frailty in patients undergoing percutaneous mitral valve repair" Mini-invasive Surgery. 4: 67. http://dx.doi.org/10.20517/2574-1225.2020.54
ACS Style
Benito-González, T.; Estévez-Loureiro R.; del Castillo S.; Minguito-Carazo C.; Echarte-Morales J.; Garrote-Coloma C.; Fernández-Vázquez F. The prognostic impact of frailty in patients undergoing percutaneous mitral valve repair. Mini-invasive. Surg. 2020, 4, 67. http://dx.doi.org/10.20517/2574-1225.2020.54
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 9 clicks
Cite This Article 9 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.