Robotic pancreaticoduodenectomy and splenopancreatectomy: technical aspects and review of literature
Abstract
Robotic pancreatic surgery provides several advantages. Since the first report of a robotic-assisted distal pancreatectomy in 2001, total pancreatectomies, pancreatic tumor enucleations, pancreaticoduodenectomy, central pancreatectomy and Appleby procedures have been performed, indicating a promising future. The aim of this article is to describe our experience of robotic pancreatic surgery including technical aspects for pancreaticoduodenectomy and distal pancreatectomy. The current literature on feasibility, safety and early postoperative outcomes will be discussed.
Keywords
Introduction
Morbidity and mortality associated with pancreatic surgery has decreased over the last decades because of advances in anesthesia, critical care and other aspects of perioperative management. Improvement in surgical technique and instrumentation as well as centralization of care to high-volume pancreatic surgery centers has significantly contributed to improvement in postoperative short- and long-term outcomes[1].
The robotic platform provides significant dexterity-related advantages, enabling pancreatic procedures to be performed with surgeon- and patient-related benefits. Complex demanding procedures such as pancreaticoduodenectomies (PDs) involving dissection of the hepatoduodenal ligament and resection of the pancreatic head, uncinate process and duodenum, followed by a complex reconstruction with delicate anastomoses become technically feasible using a minimally invasive approach[2]. Adjuncts such as built-in fluorescence imaging FireFlyTM and TileProTM picture overlay while performing intraoperative ultrasound add to operative safety and efficiency. At our high-volume center, we have performed more than 180 robotic PDs since 2012 and over 200 distal pancreatectomies with splenectomy (DPS) since 2008. We have found lower complication rates for robotic PD along with no differences in total costs when compared with the open PD, but more importantly, robotic PD may offer improved oncologic outcomes[3,4].
When starting a robotic program for pancreatic surgery, a dedicated team with prior experience in open as well as minimally invasive pancreatic surgery and, first and foremost, a structured training is the key to success[5]. During the early stages of the learning curve, proficiency in DPS should be achieved[6]. However, learning curves can be considerably diminished by appropriate training, proficient mentorship and an experienced multidisciplinary team[7-9].
The aim of this article is to describe the technical aspects of robotic PD and DPS. Our own expertise as well as the current literature on feasibility, safety and early postoperative outcomes will be discussed.
Technique of robotic pancreatic surgery (XiTM system)
Patient selection
Patient selection plays a crucial role during the early learning curve for successful robotic pancreatic surgery. Patients with a very high or very low body mass index (BMI > 40 kg/m2; BMI < 17 kg/m2), petite body habitus and relevant comorbidities, elderly frail patients and those with multiple previous abdominal surgeries should be evaluated thoroughly[10]. Patients with chronic pancreatitis, neuroendocrine tumors, cystic neoplasms, ampullary cancers and distal cholangiocarcinomas may be considered as ideal PD candidates for surgeons with juvenile robotic experience. Tumor entity, location and extent are important factors in determining whether a robotic approach is beneficial for the patient. Borderline resectable pancreatic tumors may require concomitant vascular or multi visceral resection demand for robotic expertise as well as master skills and should be avoided during the learning curve. A recent NSQIP database study comparing early postoperative outcomes for patients undergoing laparoscopic or robotic PD reported higher overall complications and conversion rates for the robotic approach if the procedure is combined with vascular or multivisceral resection[10].
Equipment and preoperative measures
As for robotic pancreas procedures using the Xi system, we recommend the use of PrograspTM forceps, fenestrated bipolar and mono-polar scissors as well. The robotic vessel sealerTM is the key device in facilitating dissection while achieving adequate hemostasis. Fortunately, a new sealing device with a more delicate articulating tip and shorter seal time is soon to be launched (SynchroSealTM). Locking robotic plastic clips (HemolokTM, WeckTM) are used prior to the division of larger vessels. Pancreatic transection may be achieved with the help of the robotic stapler. Cutting or non-cutting needle drivers may be used for reconstruction according to surgeon’s preference. A commonly used suture for our robotic pancreatic procedures is 4-0 or 5-0 Monocryl [Table 1].
Equipment for robotic pancreatic procedures
| Items | Details (number) |
|---|---|
| Robotic system | Da VinciTM Xi |
| Robotic instruments | 30-degree camera
PrograspTM Fenestrated Bipolar Mono-polar scissors Large and diamond needle drivers Bipolar vessel sealing device Large clip applier Robotic bulldog clamps Ultrasound probe |
| Ports | 12 mm assistant trocars
(4) 8 mm robotic trocars |
| Basic laparoscopic tray | Veress needle
Suction - irrigation Needle drivers Stapling devices on standby |
| Suture | 0 Vicryl suture
4-0 V-lock 4-0 Monocryl, cut to 20/15/12 cm 5-0 Monocryl, cut to 12 cm 6-0 Monocryl, cut to 12 cm |
| Specimen bags | Cook LapSacTM - 5 × 8, 8 × 10 (inches) |
| Drains | 19 French Blake drain |
Most surgical departments have designated robotic operating suites. Placement of the robotic cart, console(s), and audio/video towers in relation to the patient, scrub team and anesthesia is set up according to the surgeon’s preferences ahead of surgery. The patient table is placed at 45 degrees to the anesthesia team. Both arms are abducted, and the patient is positioned supine with slight flexion and slight reverse Trendelenburg. The robot cart docks from the right of the patient table.
Entry and port placement
Access is obtained by an infraumbilical incision and abdominal insufflation via a Veress needle followed by a 12-mm bladeless trocar insertion. In patients with previous surgery, insufflation may be obtained by placing a Veress needle in the left subcostal region in the mid clavicular line followed by entry with a 5-mm bladeless trocar and 5-mm laparoscope.
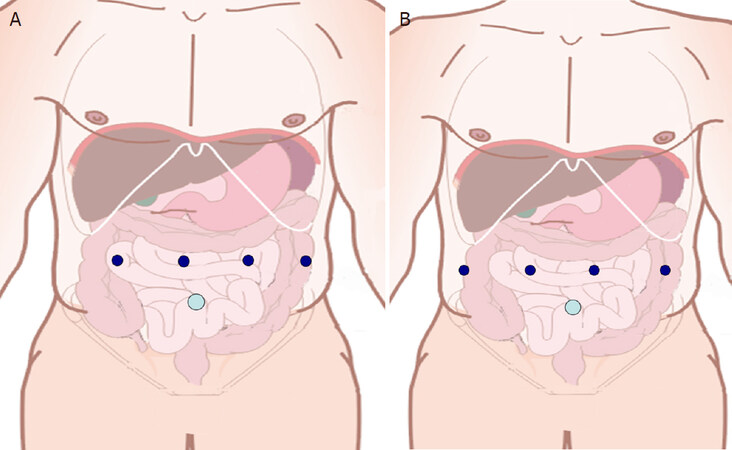
Using the Xi system, the 12-mm umbilical port is used as the assistant port. This may also serve as a robotic working port (robotic stapler). The robotic ports are placed along a straight line at variable distance from target anatomy depending on the patient’s body habitus. The robotic camera trocar is placed in the right mid-clavicular line. Two working ports are placed on the left, with one on the right at distance of 6-8 cm between each port [Figure 1A (DPS) and B (PD)]. When using the robotic stapler, the 12-mm robotic trocar is inserted at the site of the assistant port followed by bringing down arm number 3.
Distal pancreatectomy and splenectomy
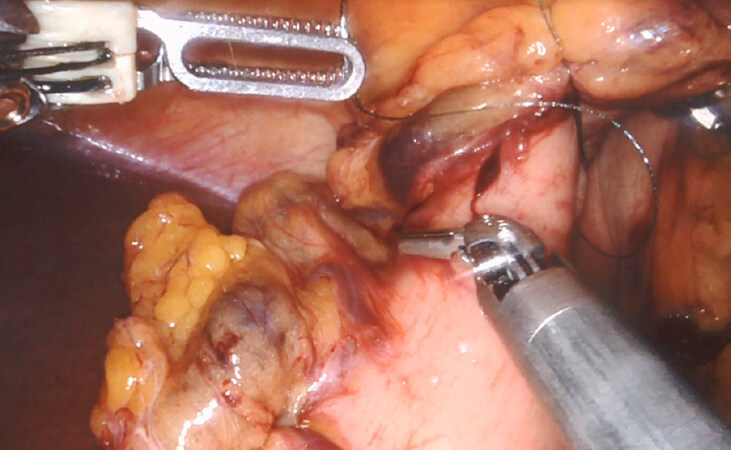
ProGraspTM and fenestrated bipolar forceps are used to enter the lesser sac. The robotic vessel sealer is used to divide the gastrocolic and splenocolic ligament. Congenital adhesions posterior between the stomach and pancreas or adhesions are released with the help of the vessel sealer. To facilitate and optimize exposure, the posterior surface of the stomach is subsequently suspended to the anterior abdominal wall with a running barbed suture [Figure 2].
Tumor location and its relation to key vascular structures are confirmed using the intraoperative ultrasound probe. The TileProTM picture overlay option enables simultaneous visualization of the ultrasound images and identification of structures in the operative field.
Next, the peritoneum overlying the inferior border of the pancreas is incised using monopolar scissors. Further dissection along the plane between the posterior aspect of the pancreas and the retroperitoneum from medial to lateral is performed. Superior mesenteric vein (SMV) and portosplenic confluence are identified as dissection and tunneling continues toward the superior border of the pancreas [Figure 3]. Robotic micro-clips are used to clip small venous branches draining directly from the pancreas into the splenic vein. The peritoneum at the superior margin of the body of the pancreas is incised. Delicate dissection to identify the splenic artery take off from the celiac trunk and concomitant lymphadenectomy is performed.
Intraoperative ultrasound and also a clamping trial using bulldogs are applied to confirm doubtless identification of the splenic artery. The artery may then be divided using locking plastic clips. The neck of the pancreas is encircled via the created tunnel with a Dacron umbilical tape. Resection continues with division of the pancreas using a stapling device (robotic or laparoscopic stapler through the assistant port). The splenic vein is isolated and divided distal to the confluence applying locking plastic clips. The pancreas is then further dissected off the retroperitoneum. The specimen is placed in the retrieval bag and removed via the umbilical port, which may be enlarged to permit specimen extraction.
Robotic radical antegrade modular pancreatosplenectomy (robotic RAMPS) may be beneficial in selected patients. The mode of dissection is also from medial to lateral; however, as a more radical approach, the left renal vein is exposed and Gerota’s fascia is cleared off the left kidney. The left adrenal is resected en bloc if the tumor breaks through the posterior plane. The dissection continues further posteriorly to the diaphragm using the retroperitoneal muscles as the posterior border, diaphragm as the superior border, and renal vein as the inferior border of the dissection plane. Radical lymphadenectomy including the gastrosplenic, splenic, infrapancreatic and gastroduodenal nodes is performed. In addition, lymph nodes along the celiac part of the aorta and superior mesenteric arteries are removed[11].
Robotic pancreaticoduodenectomy
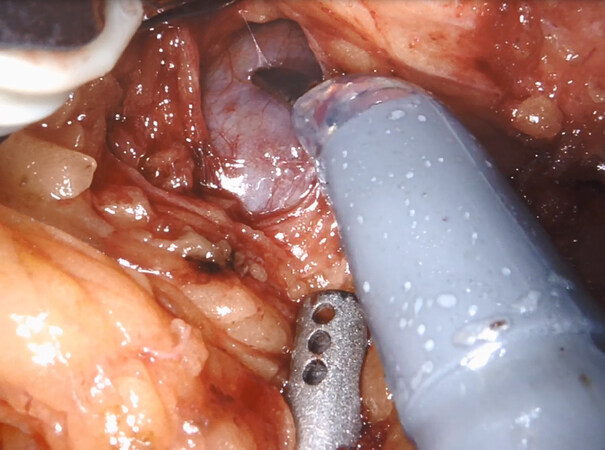
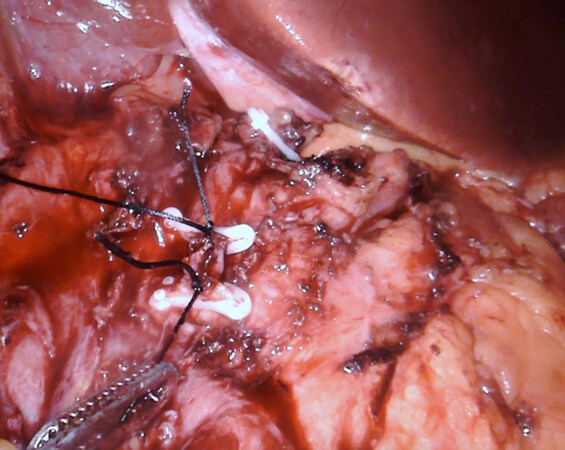
The falciform ligament is taken down and to be used as a vascularized pedicled flap[3]. To optimize surgical exposure of the hepatoduodenal ligament, the gallbladder is sutured to the anterior abdominal wall. In absence of a gallbladder a Nathanson retractor is introduced. The robotic vessel sealer is used to open the gastrocolic ligament and the distal gastric antrum as well as the proximal duodenum are dissected. The right gastroepiploic and right gastric artery are identified and divided between locking clips. The hepatic flexure of the colon is taken down and the duodenum Kocherized followed by the division of the proximal duodenum using a 60-mm robotic stapler [Figure 4].
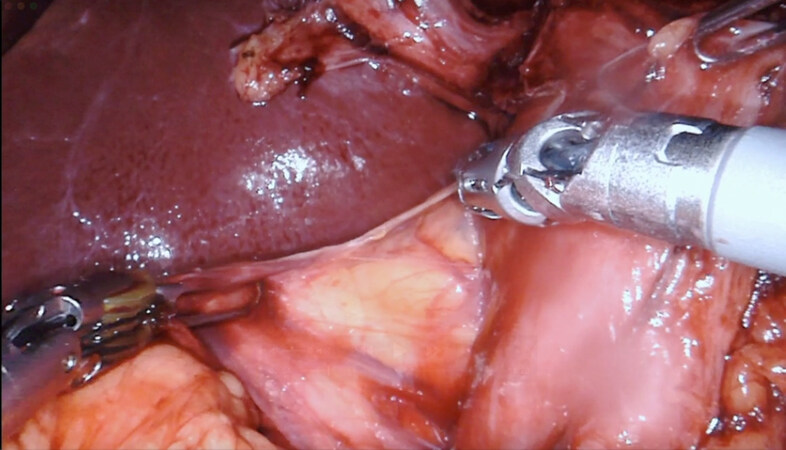
TileProTM picture overlay while performing intraoperative ultrasound is used to evaluate the vasculature prior to division of vessels. Fluorescence imaging FireFlyTM assists in identifying the biliary structures. The hepatic artery is dissected, and lymphadenectomy is performed. After identification of the gastroduodenal artery and determination of its relevance for the hepatic blood supply (clamping trial), the artery is ligated using silk sutures, clipped with HemolockTM clips and divided leaving a stump on the hepatic portion [Figure 5].
The ligament of Treitz is identified. Using a robotic stapler, the jejunum is divided 20 cm distal to the ligament of Treitz. The mesentery is transected using the vessel sealer. Further dissection from the right upper quadrant enables a pull through of the proximal jejunum.
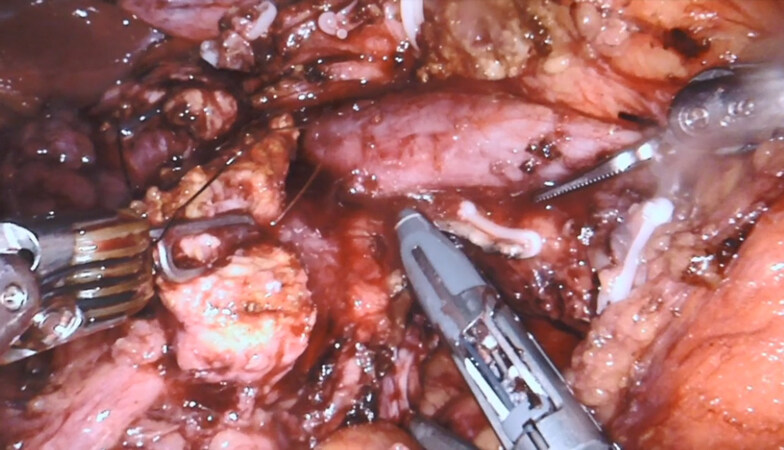
The peritoneum overlying the inferior border of the pancreas is incised, the vein of Henle (gastrocolic trunc) identified and followed towards the SMV. A tunnel between the pancreatic neck and the SMV/portal vein is created. An umbilical tape is then passed through this tunnel. Pancreatic neck transection is performed using the monopolar scissors coupled with saline irrigation. Following division of the pancreas, the uncinate process is dissected off the superior mesenteric vessels using the vessel sealer [Figure 6].
The cystic artery and duct are clipped and divided. The common hepatic duct is transected just above the take off of the cystic duct. The specimen is placed in a retrieval bag for removal at the end of surgery and meanwhile placed in the lower abdomen.
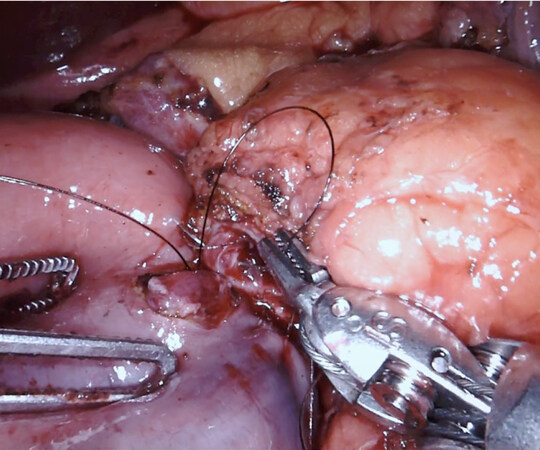
A window is created in an avascular area of the transverse mesocolon, and the jejunum is pulled through. The pancreaticojejunostomy (PJ) is performed as a two-layer end-to-side anastomosis with duct to mucosa approximation. A 4-0 monofilament running suture is used to create the posterior layer of the anastomosis. Monofilament sutures (5-0) are applied to create the duct to mucosa anastomosis in interrupted fashion [Figure 7].
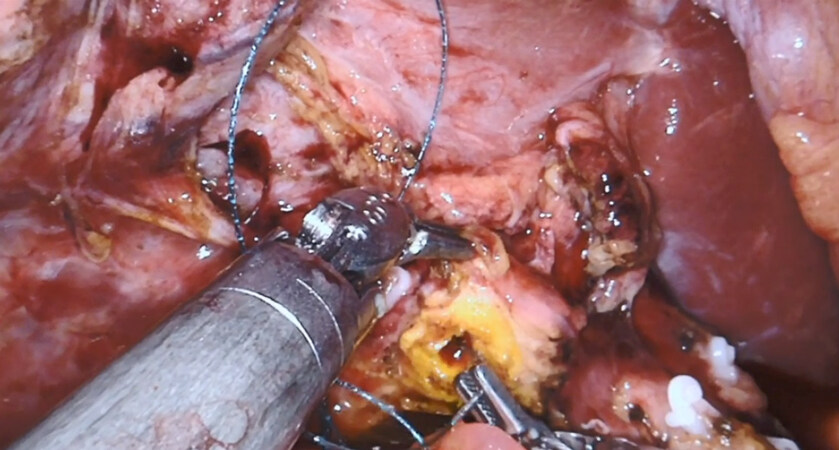
Stents may be used depending on the diameter of the pancreatic duct and consistency. After making a small enterotomy to the jejunum, the hepaticojejunostomy may be performed in a running (larger ducts, 4-0 barbed suture) or interrupted (smaller ducts, 4-0 or 5-0 monofilament) fashion 10-15 cm downstream from the PJ [Figure 8].
The duodenojejunostomy may be performed ante- or transmesocolic. An antimesenteric enterotomy is made, the anastomosis is performed in a seromuscular, in a single-layer running fashion using a barbed absorbable monofilament suture (4-0).
The vascularized falciform ligament flap is pulled through the empty space behind the pancreaticojejunostomy. A 19 French Blake drain is placed in proximity to the pancreatic and biliary anastomosis. Specimen extraction is performed via a Pfannenstiel incision at the surgeon’s discretion[2].
Discussion
Robotic pancreatic surgery provides several advantages and enables the surgeon to perform complex resections and reconstructions by facilitating supraphysiological movements with the robotic instruments[12]. Since the first report of a robotic-assisted distal pancreatectomy in 2001, total pancreatectomies, pancreatic tumor enucleations, pancreaticoduodenectomy, central pancreatectomy and Appleby procedures have been performed, indicating a promising future. Results of randomized control trials comparing robot-assisted PD with the laparoscopic or open approach are lacking. Patient recruitment for one randomized control trials in China and one in the USA started in 2020, and results are expected for 2024. The international consensus statement on robotic pancreatic surgery published last year reveals that the level of evidence still remains moderate to low for the robotic platform[13].
A review of our own experience revealed longer operative times of approximately 136 min when compared with our open PD cohort[14]. However, robotic PD resulted in less blood loss (200 mL lower), a shorter intensive care unit stay, a lower 30-day complication rate, and no difference in total costs compared with open PD[15,16].
Perhaps more importantly, we found that with increasing experience, the pancreatic fistula rate could be reduced to below that of most open as well as laparoscopic series (7.4% vs. 12%) and that the robotic approach may offer improved oncologic outcomes.
The significantly higher lymph node yield and decreased inflammatory response demonstrated in robotic surgery may improve overall survival[4,17].
Multiple single or multi-institutional retrospective studies to compare specific outcomes between robotic, laparoscopic and open approaches are reported[6,14-16,18-24]. A large systematic review examined data from 13 retrospective series[25]. It compared the outcomes of 738 patients who underwent robotic and open PDs between 2000 and 2016. The data showed that the robotic approach was associated with longer operative times but lower estimated blood loss. The learning curve to decrease rates of conversion to an open procedure was found to be as high as 20 robotic PDs. Overall morbidity rates were comparable between the robot and open groups. Mortality rates also did not differ between the two approaches and ranged between 1%-12.5%. Delayed gastric emptying, however, was found to be lower with the robotic approach[25,26]. An NSQIP study comparing 30-day outcomes between laparoscopic and robotic PDs found that there was no difference in 30-day morbidity or mortality between the two approaches[10]. However, they did find that the rates of conversion to an open procedure were higher for patients undergoing laparoscopic PD (26% vs. 11.3%).
Increasing proficiency with robotic pancreatic surgery is reflected in a decrease in operative times as well as conversion rates. Other more sophisticated factors may include number of lymph nodes resected, blood loss, R-status, hospital stay, and 90-day complications and readmission as well[8]. Our initial experience with robotic pancreatic surgery revealed a conversion rate of one in four decreasing to one in 32 cases after overcoming the learning curve. In line with this, procedural duration decreased significantly over time. Boone et al.[12] reported that blood loss and conversion rate decrease significantly after 20 robotic PD cases. The clinically relevant Grade B/C pancreatic fistulas rate (POPF) decreased by half after 40 cases along with a significant decrease in operative times after 80 cases.
While laparoscopic skills enhance the learning curve in our experience, training in robotic surgery should be structured. In a first phase basic skills and procedure specific skills with the help of simulation, biotissue drills, video libraries, live case observations, and training courses have to be achieved[27]. The second phase consists of fellowships, and proctoring programs to ensure patient safety during the first procedures. During the third phase the surgeon’s aim is to safely implement the procedure into standard practice, while minimizing the learning curve related to excess morbidity and mortality. Adequate training and high procedural volume are key to implementing robotic pancreatic surgery safely[28].
Conclusion
Robotic hepatopancreatobiliary surgery has undergone rapid evolvement over the last two decades. Its adoption has been tempered by the complexity of the procedures. The combination of superior articulation, better optics and elimination of tremor provides technical and ergonomic advantages over conventional laparoscopy. At high-volume centers, once the learning curve has been surpassed, robotic PD has been shown to be non-inferior to open PD in terms of POPF development and other perioperative outcomes. The higher operative cost of the procedure may be offset by lower hospital length of stays associated with a minimally invasive approach. However, more robust data in the form of a randomized controlled trial and other cost benefit studies are needed.
Declarations
Authors’ contributionsMade substantial contributions to the design of the work, interpretation of data, and drafting and substantive revision of the manuscript: Tschuor C, Nagarkatti SS, Salibi PN, Vrochides D, Martinie JB
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestJohn B. Martinie is a proctor for Intuitive. Christoph Tschuor’s fellowship salary is granted by Intuitive.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2020.
REFERENCES
1. Mackay TM, Smits FJ, Latenstein AEJ, Bogte A, Bonsing BA, et al. Impact of nationwide enhanced implementation of best practices in pancreatic cancer care (PACAP-1): a multicenter stepped-wedge cluster randomized controlled trial. Trials 2020;21:334.
2. Nagarkatti SS, Sastry AV, Vrochides D, Martinie JB. How i do it: robotic pancreaticoduodenectomy. J Gastrointest Surg 2019;23:1672-81.
3. Sola R, Kirks RC, Iannitti DA, Vrochides D, Martinie JB. Robotic pancreaticoduodenectomy. J Vis Surg 2016;2:126.
4. Baimas-George M, Watson M, Murphy KJ, Iannitti D, Baker E, et al. Robotic pancreaticoduodenectomy may offer improved oncologic outcomes over open surgery: a propensity-matched single-institution study. Surg Endosc 2020;34:3644-9.
5. Nota CL, Zwart MJ, Fong Y, Hagendoorn J, Hogg ME, et al. Developing a robotic pancreas program: the Dutch experience. J Vis Surg 2017;3:106.
6. Napoli N, Kauffmann EF, Menonna F, Costa F, Iacopi S, et al. Robotic versus open pancreatoduodenectomy: a propensity score-matched analysis based on factors predictive of postoperative pancreatic fistula. Surg Endosc 2018;32:1234-47.
7. Tsamalaidze L, Stauffer JA. Pancreaticoduodenectomy: minimizing the learning curve. J Vis Surg 2018;4:64.
8. Vining CC, Hogg ME. How to train and evaluate minimally invasive pancreas surgery. J Surg Oncol 2020;122:41-8.
9. Jones LR, Zwart MJW, Molenaar IQ, Koerkamp BG, Hogg ME, et al. Robotic pancreatoduodenectomy: patient selection, volume criteria, and training programs. Scand J Surg 2020;109:29-33.
10. Nassour I, Wang SC, Porembka MR, Yopp AC, Choti MA, et al. Robotic versus laparoscopic pancreaticoduodenectomy: a NSQIP Analysis. J Gastrointest Surg 2017;21:1784-92.
11. Trottman P, Swett K, Shen P, Sirintrapun J. Comparison of standard distal pancreatectomy and splenectomy with radical antegrade modular pancreatosplenectomy. Am Surg 2014;80:295-300.
12. Boone BA, Zenati M, Hogg ME, Steve J, Moser AJ, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 2015;150:416-22.
13. Liu R, Wakabayashi G, Palanivelu C, Tsung A, Yang K, et al. International consensus statement on robotic pancreatic surgery. Hepatobiliary Surg Nutr 2019;8:345-60.
14. Kauffmann EF, Napoli N, Menonna F, Iacopi S, Lombardo C, et al. A propensity score-matched analysis of robotic versus open pancreatoduodenectomy for pancreatic cancer based on margin status. Surg Endosc 2019;33:234-42.
15. Zhao W, Liu C, Li S, Geng D, Feng Y, et al. Safety and efficacy for robot-assisted versus open pancreaticoduodenectomy and distal pancreatectomy: a systematic review and meta-analysis. Surg Oncol 2018;27:468-78.
16. Watkins AA, Kent TS, Gooding WE, Boggi U, Chalikonda S, et al. Multicenter outcomes of robotic reconstruction during the early learning curve for minimally-invasive pancreaticoduodenectomy. HPB (Oxford) 2018;20:155-65.
17. Baker EH, Ross SW, Seshadri R, Swan RZ, Iannitti DA, et al. Robotic pancreaticoduodenectomy: comparison of complications and cost to the open approach. Int J Med Robot 2016;12:554-60.
18. Ielpo B, Caruso R, Duran H, Diaz E, Fabra I, et al. Robotic versus standard open pancreatectomy: a propensity score-matched analysis comparison. Updates Surg 2019;71:137-44.
19. Liu R, Liu Q, Zhao ZM, Tan XL, Gao YX, et al. Robotic versus laparoscopic distal pancreatectomy: a propensity score-matched study. J Surg Oncol 2017;116:461-9.
20. Liu R, Zhao GD, Tang WB, Zhang K, Zhao ZM, et al. A single-team experience with robotic pancreatic surgery in 1010 cases. Nan Fang Yi Ke Da Xue Xue Bao 2018;38:130-4. (in Chinese)
21. Souche R, Herrero A, Bourel G, Chauvat J, Pirlet I, et al. Robotic versus laparoscopic distal pancreatectomy: a French prospective single-center experience and cost-effectiveness analysis. Surg Endosc 2018;32:3562-9.
22. Qu L, Zhiming Z, Xianglong T, Yuanxing G, Yong X, et al. Short- and mid- term outcomes of robotic versus laparoscopic distal pancreatosplenectomy for pancreatic ductal adenocarcinoma: A retrospective propensity score-matched study. Int J Surg 2018;55:81-6.
23. Kim HS, Han Y, Kang JS, Kim H, Kim JR, et al. Comparison of surgical outcomes between open and robot-assisted minimally invasive pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2018;25:142-9.
24. Lyman WB, Passeri M, Sastry A, Cochran A, Iannitti DA, et al. Robotic-assisted versus laparoscopic left pancreatectomy at a high-volume, minimally invasive center. Surg Endosc 2019;33:2991-3000.
25. Kornaropoulos M, Moris D, Beal EW, Makris MC, Mitrousias A, et al. Total robotic pancreaticoduodenectomy: a systematic review of the literature. Surg Endosc 2017;31:4382-92.
26. McMillan MT, Zureikat AH, Hogg ME, Kowalsky SJ, Zeh HJ, et al. A propensity score-matched analysis of robotic vs open pancreatoduodenectomy on incidence of pancreatic fistula. JAMA Surg 2017;152:327-35.
27. Hogg ME, Besselink MG, Clavien PA, Fingerhut A, Jeyarajah DR, et al. Training in minimally invasive pancreatic resections: a paradigm shift away from “See one, Do one, Teach one.”. HPB (Oxford) 2017;19:234-45.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Tschuor C, Nagarkatti SS, Salibi PN, Vrochides D, Martinie JB. Robotic pancreaticoduodenectomy and splenopancreatectomy: technical aspects and review of literature. Mini-invasive Surg 2020;4:72. http://dx.doi.org/10.20517/2574-1225.2020.39
AMA Style
Tschuor C, Nagarkatti SS, Salibi PN, Vrochides D, Martinie JB. Robotic pancreaticoduodenectomy and splenopancreatectomy: technical aspects and review of literature. Mini-invasive Surgery. 2020; 4: 72. http://dx.doi.org/10.20517/2574-1225.2020.39
Chicago/Turabian Style
Tschuor, Christoph, Sushruta S. Nagarkatti, Patrick N. Salibi, Dionisios Vrochides, John B. Martinie. 2020. "Robotic pancreaticoduodenectomy and splenopancreatectomy: technical aspects and review of literature" Mini-invasive Surgery. 4: 72. http://dx.doi.org/10.20517/2574-1225.2020.39
ACS Style
Tschuor, C.; Nagarkatti SS.; Salibi PN.; Vrochides D.; Martinie JB. Robotic pancreaticoduodenectomy and splenopancreatectomy: technical aspects and review of literature. Mini-invasive. Surg. 2020, 4, 72. http://dx.doi.org/10.20517/2574-1225.2020.39
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.