Therapeutic EUS
Abstract
Currently, the standard treatment for pancreatic neoplasms is surgical resection. However, pancreatic surgical resection is associated with high morbidity and mortality. Patients unfit for surgery are undergoing regular cross-sectional imaging surveillance. Controversy surrounds the optimal surveillance of patients with pancreatic neoplasms, underlying the need for minimally invasive treatment modalities as an alternative to surgical treatment. To date, endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) is an emerging minimally invasive therapeutic alternative to surgical resection for various pancreatic neoplasms. We review evaluations of EUS-RFA for various pancreatic neoplasms to better understand its effectiveness and safety.
Keywords
INTRODUCTION
Endoscopic ultrasound (EUS) is widely used to diagnose biliopancreatic diseases. The development of EUS-guided fine needle aspiration (EUS-FNA) in the early 1990s has expanded EUS-guided interventions. After the development of the linear echoendoscope and the increasing sizes of the EUS devices’ working channels, EUS has recently evolved to become a therapeutic method for patients with biliopancreatic disease[1].
Although surgical resection has been the only curative treatment for various pancreatic tumors, pancreatic surgery is related with high morbidity and mortality[2,3]. The recent advance of the EUS device has led to an increase in EUS-guided local treatment for pancreatic tumors. Radiofrequency ablation (RFA) is a local treatment that uses heat energy generated by the agitation of ions in cells to induce cell death and coagulation necrosis[4]. RFA has been widely used to treat solid tumors in organs such as the liver, lungs, and kidneys. Recently, EUS-RFA has been described as an effective and safe new therapeutic modality for treating pancreatic neoplasms. We review EUS-RFA for pancreatic neoplasms and its outcomes.
POTENTIAL INDICATIONS
Currently, there are no established indications of EUS-RFA. However, EUS-RFA can be used for various tumors, including benign solid pancreatic tumors, such as neuroendocrine tumors and insulinomas[5-7], pancreatic cystic tumors[6], and pancreatic cancers[8,9]. There is no absolute contraindications of EUS-RFA. However, as previously reported, there is the possibility that severe adverse events may develop when EUS-RFA is applied to pancreatic lesions close to the main pancreatic duct[5,10]. Therefore, it may be considered as a relative contraindication of EUS-RFA.
MATERIALS AND INSTRUMENTS
1. An oblique-viewing therapeutic curvilinear array echoendoscope.
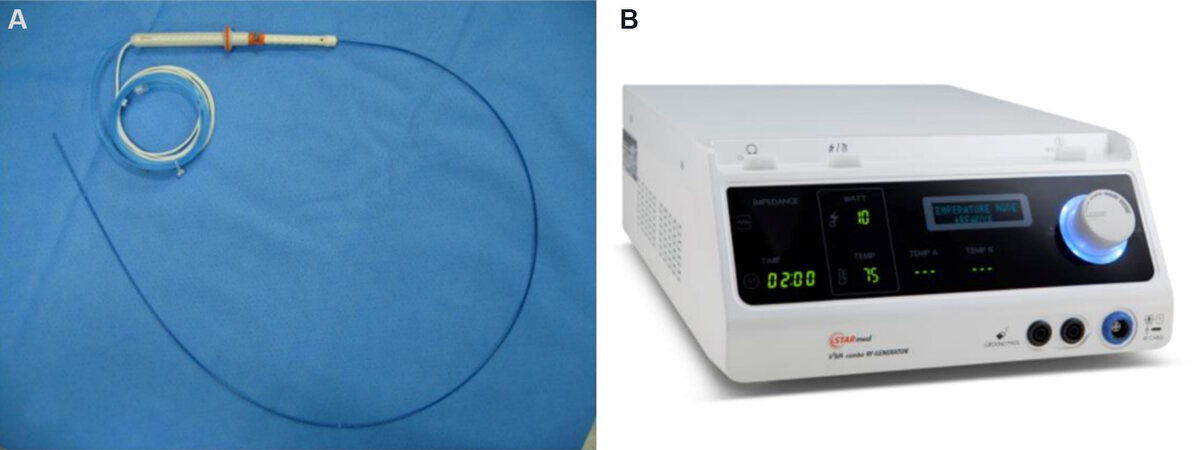
2. Radiofrequency (RF) generator [Figure 1].
Figure 1. Endoscopic radiofrequency electrode and power generator (STARmed, Koyang, Korea): 19-gauge endoscopic radiofrequency ablation electrode (A); and a VIVA radiofrequency power generator (B).
3. Radiofrequency ablation (RFA) probes: The currently available RFA probes are EUSRA RF electrodes (STARmed, Koyang, Korea), HabibTM EUS-RFA catheters (EMcision Ltd., London, UK), 19-gauge EUS-FNA needle electrodes (Radionics, Inc., Burlington, MA, USA), and hybrid cryotherm probes (Hybrid-Therm®; ERBE, Tübingen, Germany). EUS-RFA probes are classified as “through the needle” type and “EUS-RFA needle” type. Habib EUS-RFA catheter is a “through the needle” type and the remaining three probes are “EUS-RFA needle” types. Among these probes, the Hybrid cryothermal probe is bipolar and the rest are monopolar probes. All probes are connected to the RF generator to deliver heat energy to the target lesions.
4. Ultrasound contrast agents (UCAs): UCAs are useful for identifying remnant tumors, evaluation of early treatment responses, and an accurate guidance for additional ablation[11].
TECHNIQUE
Prophylactic antibiotics are administered intravenously before EUS-RFA. An RFA probe is inserted into the target lesion under EUS-guidance to avoid major vessels or the pancreatic or bile ducts [Figure 2A]. Ablation is usually started at the far end inside the lesion [Figure 2B]. After the needle tip is identified within the margin of the tumor on EUS, the RF generator is activated to deliver energy [Figure 2C]. After a lag period, echogenic bubbles gradually start appearing around the needle, indicating effective ablation at the site [Figure 2D]. The size of the ablation zone depends on the wattage, RFA needle tip length, and time duration. For the ablation of large lesions, the electrode may be repositioned under EUS visualization to ablate another zone along the same trajectory closer to the echoendoscope while staying away from the gut wall. A fanning technique allows additional needle passes to further ablate different areas within the same lesion.
Figure 2. Image of EUS-RFA: a 19-gauge needle probe is inserted into the pancreatic tumor under EUS-guidance (A); ablation is usually started in the right distal part of the tumor on the EUS image at the far end inside the lesion (B); echogenic bubbles are identified around the needle after ablation (C); and after needle withdrawal and reinsertion into the mass, RFA is repeated on the left side of the previous ablation site (D). EUS-RFA: Endoscopic ultrasound-guided radiofrequency ablation.
RFA-related adverse events are closely related with thermal injury to the pancreatic parenchyma and surrounding structures, including blood vessels, bile ducts, the stomach, and the duodenum. Technical precautions are required for preventing thermal injury to adjacent organs, including maintenance of a
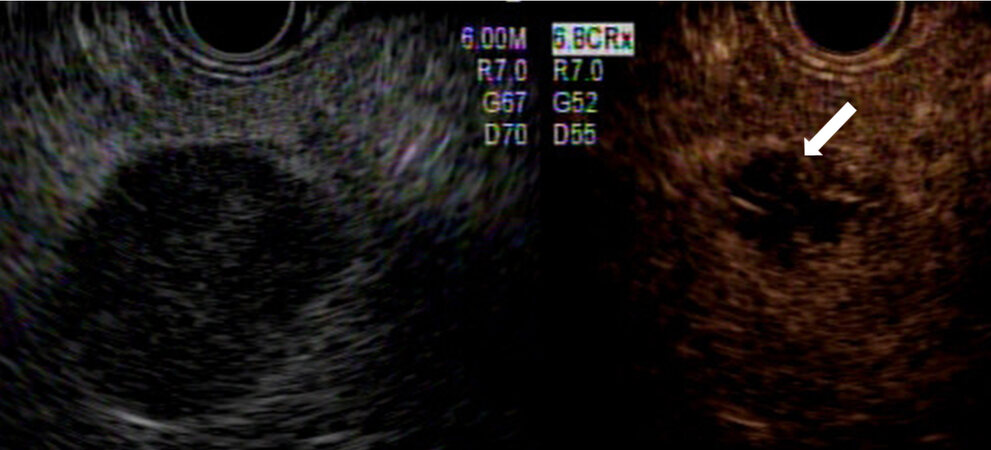
After the initial session of EUS-RFA, early treatment response can be evaluated by contrast-enhanced-EUS (CE-EUS)[11]. CE-EUS is helpful for differentiating viable tumors after ablation and targeting remnant viable tumors. When a viable tumor is identified on the CE-EUS, a second RFA session can ablate remnant tumors [Figure 3].
DISCUSSION
Outcomes of EUS-RFA in benign solid pancreatic tumor
Since Goldberg et al.[12] first reported EUS-RFA for the pancreas in a porcine model in 1999, several studies have demonstrated its effectiveness for various pancreatic tumors. Table 1 summarizes the clinical outcomes of previous research.
Summary of published data on EUS-RFA
| Ref. (year) | Indications and number of patients | RF devices | Mean tumor size (range) | Application power and time | Mean RF sessions | Technical success | Treatment response | Follow-up periods | Adverse events |
| Pai et al.[19] (2015) | Mucinous cyst (4), IPMN (1), microcystic adenoma (1), NET (2) | Habib EUS-RFA catheter | Pancreas cyst: 36.5 (24-70), NET: 27.5 (15-40) | 5-25W, 90-120s | 1.3 (1-2) | 100% | Pancreas cyst: complete resolution (2, 33%), size reduction (4, 67%), NET: 50% reduction with vascular changes (2, 100%) | 3-6 months | Mild abdominal pain (2, 25%) |
| Lakhtakia et al.[7] (2016) | Insulinoma (3) | EUSLA | 19 (14-22) | 50W, 10-15s | 1 | 100% | Complete resolution of hypoglycemia (3, 100%) | 11-12 months | None |
| Song et al.[8] (2016) | Locally advanced pancreatic cancer (4), metastatic pancreatic cancer (2) | EUSLA | 38 (30-90) | 20-50W, 10s | 1.3 (1-2) | 100% | Necrosis at the ablation site (6, 100%) | 2-6 months | Mild abdominal pain (2, 33%) |
| Scopelliti et al.[9] (2018) | Locally advanced pancreatic cancer (10) | EUSLA | 49.2 (35-75) | 20-30W, 100-560s | 1.4 (1-2) | 100% | Necrosis at the ablation site (10, 100%) | 30 days | Mild abdominal pain (2, 20%), ascites (2, 20%), peripancreatic effusion (2, 20%) |
| Choi et al.[5] (2018) | NET (7), solid pseudopapillary neoplasm (2), insulinoma (1) | EUSLA | 20 (8-28) | 50W | 1.6 (1-3) | 100% | Radiologic complete response (7, 70%) | Median 13 months | Mild abdominal pain (1, 10%), acute pancreatitis (1, 10%) |
| Barthet et al.[13] (2019) | IPMN (16), MCN (1), NET (14 lesions in 12) | EUSLA | PCL: 28 (9-60), NET: 13.1 (10-20) | 50W | NA | 100% | NET: radiologic complete response (12, 86%) Pancreas cyst: complete response (11, 65%), more than 50% reduction (1, 6%) | 12 months | Acute pancreatitis (1, 3%), jejunal perforation (1, 3%), main pancreatic duct obstruction (1, 3%) |
| Oleinikov et al.[14] (2019) | NET (18 lesions in 11 patients), insulinoma (9 lesions in 7 patients) | EUSLA | 14.3 (4.5-30) | 10-50W, 5-12s | NET: radiologic complete response (17 lesions, 94%) Insulinoma: complete resolution of hypoglycemia with normalization of glucose levels (7 patients, 100%) | Mean 8.7 ± 4.6 months (range 2-21 months) | Acute pancreatitis (2, 11%) | ||
| Oh et al.[20] (2020) | Microcystic SCN (13) | EUSLA | 50 (34-52.5) | 50W | 1.46 (1-2) | 100% | Partial response (8, 61.5%) | Median 9.21 months (IQR 4.79-32.39) | Abdominal pain (1, 7%) |
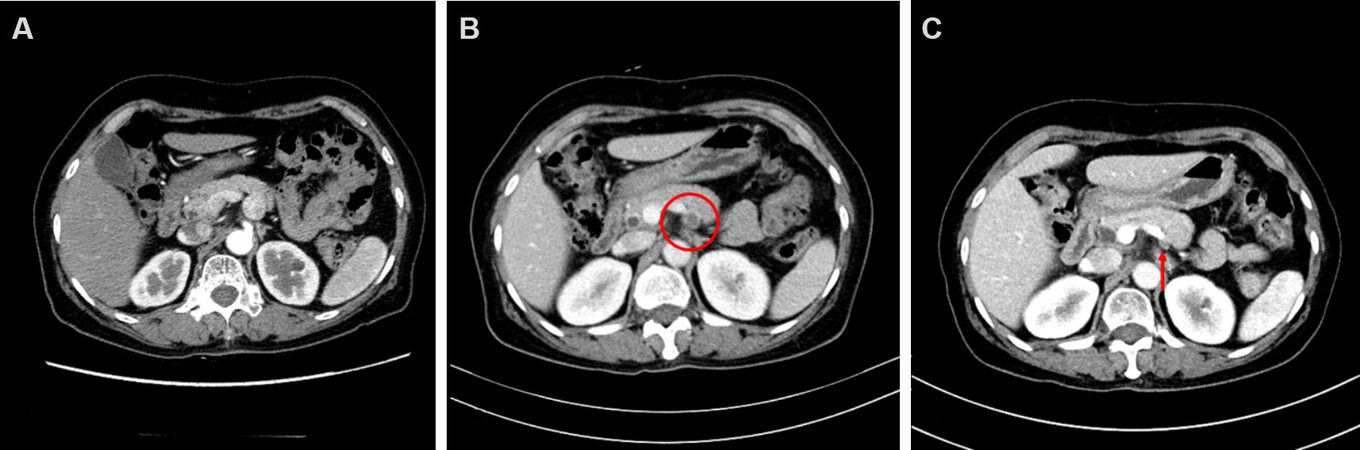
In a study by Lakhtakia et al.[7], EUS-RFA was performed in three patients with symptomatic pancreatic insulinoma using an internally cooled prototype needle electrode (EUSRA, STARmed). After ablation of pancreatic insulinoma, symptomatic relief with biochemical improvement was achieved in all patients, and patients were followed-up without symptoms for 12 months. In a prospective study by Choi et al.[5], 10 patients with benign solid pancreatic tumors [nonfunctional neuroendocrine tumor (NET), n = 7; solid pseudopapillary neoplasm, n = 2; and insulinoma, n = 1] underwent EUS-RFA. After 16 EUS-RFA sessions, a radiologic complete response was identified in seven patients during a median follow-up of 13 months [Figure 4].
Figure 4. Computed tomography (CT) images of a neuroendocrine tumor in the body of pancreas: before treatment, 20-mm hyperenhacing lesion (A); at 3-month follow-up, CT scan showing decreased peripheral rim enhancing lesion (red circle) (B); and at
In a study by Barthet et al.[13], 12 patients with 14 NET underwent EUS-RFA. At the 1-year follow-up, 12 NETs showed complete response or lesion necrosis (86%). Oleinikov et al.[14] performed ablation on 18 patients (NET, n = 11; and insulinoma, n = 7) with 27 lesions. All patients with insulinoma demonstrated complete relief of hypoglycemia-associated symptoms and normalization of glucose levels was observed 1 h after RFA. Radiologic complete response was achieved in 96.3% of patients (17 of 18) during a median of 8.7 months without clinically significant recurrence. In 2020, de Nucci et al.[15] reported a prospective study on EUS-RFA in 10 patients with 11 NETs. At the 12-month follow-up, all lesions demonstrated complete disappearance with radiological normalization. A systemic review of published research on EUS-RFA for NETs, including 12 studies and 61 patients with 73 tumors, showed an overall effectiveness rate of 96% (75%-100%) in a mean follow-up period of 11 months (1-34 months) and an adverse event rate of 13.7%, with no serious adverse events[16]. In this review, a larger tumor was related with treatment failure (mean size in the non-response group was 21.8 ± 4.71 mm vs. a mean size of 15.07 ± 7.34 mm in the response group, P = 0.048). According to the ROC curve, a NET of size ≤ 18 mm at EUS was associated with a positive response to EUS-RFA, with a sensitivity of 80% (95%CI: 28.4%-99.5%), a specificity of 78.6% (95%CI: 63.2%-89.7%), and an AUC of 0.81 (95%CI: 0.67-0.95). EUS-RFA is an effective and safe treatment for benign pancreatic tumors. However, the long-term outcomes are not well described. As solid pancreatic tumors are slow to grow and have the potential of malignant transformation, long-term follow-up data are mandatory to evaluate the outcomes of EUS-RFA.
Currently, EUS-guided ethanol ablation is most commonly used for treating pancreas cystic lesions. Although the application of EUS-guided ethanol ablation for solid pancreatic tumors is limited, few reports have demonstrated that EUS-guided ethanol ablation is effective and safe for treating benign solid pancreatic tumors. In a study by Paik et al.[17], 8 patients with borderline malignant pancreatic tumors underwent EUS-guided ethanol ablation (insulinoma, n = 3; nonfunctioning NET, n = 2; solid pseudopapillary neoplasm, n = 2; and insulinoma, n = 1). After ethanol ablation, 6 patients (75%) achieved treatment success. However, 2 patients still had persistent tumors. One patient experienced severe pancreatitis after ablation. Among 6 patients who achieved initial treatment success, 1 patient experienced tumor recurrence within 15 months. In a recent prospective study by Choi et al.[18], 33 patients who had 40 pathologically confirmed pancreatic NET (< 2 cm in diameter) underwent 63 sessions of EUS-guided ethanol-lipiodol ablation. Complete ablation was achieved in 24 of 40 tumors (60%), with 1 (18 tumors, 45%) or 2 (24 tumors, 60%) sessions of EUS-ELA. Two cases (3.4%) of pancreatitis occurred during 63 ablation procedures. There was no recurrence or progression during a median follow-up period of 42 months (IQR, 39-46 months) in patients who were successfully treated.
Currently, there is no comparative study between EUS-RFA and EUS-guided ethanol ablation for treating benign solid pancreatic tumors. In our experience, these EUS-guided treatments show similar efficacy for ablation of small (< 2 cm) pancreatic tumors. However, some technical issues remain that require further investigation, including the choice of target area and adequate ethanol dosage to achieve successful ablation without causing serious adverse events for EUS-guided ethanol ablation. Furthermore, assuming that the tumor is spherical, ethanol cannot disperse evenly into the tumors for ablation of large tumors (> 2 cm); therefore, treatment effect cannot be predicted. On the other hand, ablation area could be determined by the operator during EUS-RFA. Therefore, for ablation of large tumors, EUS-RFA is preferred to EUS-guided ethanol ablation.
Outcomes of EUS-RFA in pancreas cystic lesion
To date, there have been few studies published on EUS-RFA for treating patients with pancreatic cystic lesions (PCLs). In a prospective study by Pai et al.[19], 6 patients with benign pancreatic neoplasms (PCLs,
Outcomes of EUS-RFA in pancreatic cancer
EUS-RFA has been used for treating patients with pancreatic cancer. Song et al.[8] conducted a median of 1.3 sessions of EUS-RFA on 6 patients with unresectable pancreatic cancer. EUS-RFA was successful in all patients, and 2 patients experienced mild abdominal pain without serious adverse events. In a recent study by Scopelliti et al.[9], EUS-RFA combined with systemic chemotherapy was performed in 10 patients with unresectable pancreatic cancer. After tumor ablation, an abdominal computed tomography 30 days post-procedure revealed a delineated hypodense ablated area within the tumor in all patients. Although the role of EUS-RFA on pancreatic cancer is still being investigated, RFA may induce a secondary anticancer immune response by activating tumor-specific T lymphocytes and heat shock protein 70 expression[21,22]. Thermal ablation could increase blood flow in the ablated tissues[8]. EUS-RFA could affect post-procedural tumor changes associated with a systemic antitumor immune response, enhancing the systemic chemotherapy effect.
SUMMARY
The recent development of EUS devices has expanded the role of local treatment of EUS in pancreatic tumors. EUS-RFA may be a definite treatment for benign pancreatic tumors. EUS-RFA for pancreatic cancer could reduce tumor size, enhance the chemotherapeutic effect, and improve survival in cases of advanced pancreatic cancer. Given the promising results of previous reports, EUS-RFA can potentially change the clinical management of pancreatic neoplasms. Large-scale, prospective, randomized controlled trials are required to verify the role of EUS-guided ablation in pancreatic neoplasms.
DECLARATIONS
Authors’ contributionsConception and design: Seo DW
Analysis and interpretation of the data: Cho SH, Oh D
Drafting of the article: Cho SH, Oh D
Critical revision of the manuscript for important intellectual content: Seo DW
Supervision: Seo DW
Final approval of the article: Seo DW
Availability of data and materialsNot applicable
Financial support and sponsorshipNone
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Vilmann P, Jacobsen GK, Henriksen FW, Hancke S. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc 1992;38:172-3.
2. Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med 2004;351:1218-26.
3. Allen PJ, D'Angelica M, Gonen M, Jaques DP, Coit DG, Jarnagin WR, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg 2006;244:572-82.
4. Hwang JS, Joo HD, Song TJ. Endoscopic ultrasound-guided local therapy for pancreatic neoplasms. Clin Endosc 2020;53:535-40.
5. Choi JH, Seo DW, Song TJ, et al. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy 2018;50:1099-104.
6. Crinò SF, D'Onofrio M, Bernardoni L, et al. EUS-guided radiofrequency ablation (EUS-RFA) of solid pancreatic neoplasm using an 18-gauge needle electrode: feasibility, safety, and technical success. J Gastrointestin Liver Dis 2018;27:67-72.
7. Lakhtakia S, Ramchandani M, Galasso D, et al. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest Endosc 2016;83:234-9.
8. Song TJ, Seo DW, Lakhtakia S, et al. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc 2016;83:440-3.
9. Scopelliti F, Pea A, Conigliaro R, et al. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg Endosc 2018;32:4022-8.
10. Rossi S, Viera FT, Ghittoni G, et al. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas 2014;43:938-45.
11. Choi JH, Seo DW, Song TJ, et al. Utility of contrast-enhanced harmonic endoscopic ultrasound for the guidance and monitoring of endoscopic radiofrequency ablation. Gut and liver 2020;14:826-32.
12. Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc 1999;50:392-401.
13. Barthet M, Giovannini M, Lesavre N, et al. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy 2019;51:836-42.
14. Oleinikov K, Dancour A, Epshtein J, et al. Endoscopic ultrasound-guided radiofrequency ablation: A new therapeutic approach for pancreatic neuroendocrine tumors. J Clin Endocrinol Metab 2019;104:2637-47.
15. Nucci G, Imperatore N, Mandelli ED, di Nuovo F, d'Urbano C, Manes G. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic neuroendocrine tumors: a case series. Endosc Int Open 2020;8:E1754-e8.
16. Imperatore N, de Nucci G, Mandelli ED, et al. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic neuroendocrine tumors: a systematic review of the literature. Endosc Int Open 2020;8:E1759-e64.
17. Paik WH, Seo DW, Dhir V, Wang HP. Safety and efficacy of EUS-guided ethanol ablation for treating small solid pancreatic neoplasm. Medicine 2016;95:e2538.
18. Choi JH, Park DH, Kim MH, et al. Outcomes after endoscopic ultrasound-guided ethanol-lipiodol ablation of small pancreatic neuroendocrine tumors. Dig Endosc 2018;30:652-8.
19. Pai M, Habib N, Senturk H, et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg 2015;7:52-9.
20. Oh D, Ko SW, Seo DW, et al. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic microcystic serous cystic neoplasms: a retrospective study. Endoscopy 2020; doi: 10.1055/a-1250-7786.
21. Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol 2011;2011:160250.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Cho SH, Oh D, Seo DW. Therapeutic EUS. Mini-invasive Surg 2021;5:20. http://dx.doi.org/10.20517/2574-1225.2021.11
AMA Style
Cho SH, Oh D, Seo DW. Therapeutic EUS. Mini-invasive Surgery. 2021; 5: 20. http://dx.doi.org/10.20517/2574-1225.2021.11
Chicago/Turabian Style
Cho, Sung Hyun, Dongwook Oh, Dong-Wan Seo. 2021. "Therapeutic EUS" Mini-invasive Surgery. 5: 20. http://dx.doi.org/10.20517/2574-1225.2021.11
ACS Style
Cho, SH.; Oh D.; Seo D.W. Therapeutic EUS. Mini-invasive. Surg. 2021, 5, 20. http://dx.doi.org/10.20517/2574-1225.2021.11
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 9 clicks
Cite This Article 9 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.