An investigative review on the current role and outcomes of salvage radical cystectomy
Abstract
Salvage radical cystectomy (SRC) is currently performed after failure of a trimodal treatment (TMT) for muscle invasive bladder cancer (MIBC) and also as a palliative surgery to manage bladder cancer-related symptoms. We reviewed the available literature to assess the current outcomes of SRC. A comprehensive research of the Medline and Embase databases was carried out by following the Preferred Items for Systematic Reviews and Meta-Analysis. Bladder cancer, radiotherapy, salvage, and cystectomy were the main keywords used in the research. Due to the lack of studies, no time restriction was applied, however only English language and only studies using Clavien-Dindo Grade (CCS) to report complications were considered. Overall, 285 studies were identified, of which 41 studies were considered eligible for the purpose of this review. No comparative studies were found between TMT plus SRC and immediate radical cystectomy. Thirteen studies reported oncological outcomes after TMT. The five-year mean disease free survival rate of patients who underwent SRC after TMT was reported to be about 50% and the 5-year OS rate was between 33% and 48%. Three studies including fewer than 20 patients performed SRC with palliative purpose. Although no perioperative death occurred, patients were highly selected. Overall, 4 studies graded surgery-related complications by CCS. The rate of major complications, defined as CCS ≥ 3, was reported to be between 16% and 32%, most of them being gastrointestinal complications. SRC still preserves a role in the management of MIBC, being part of TMT and palliative care in highly selected patients. However, this surgery is at higher risk of complications and is associated with incontinent urinary diversion, thus an accurate discussion during patient counseling is advisable.
Keywords
INTRODUCTION
Nowadays, the improvements in medical, surgical, and anesthetic techniques have dramatically reduced the morbidity and mortality associated with radical cystectomy (RC), but it is still considered a major surgery with a 0.7%-42% risk of developing high-grade complications (defined as Clavien-Dindo Grade ≥ 3) and 0.4%-7% mortality rate[1].
RC is the standard treatment for muscle invasive bladder cancer (MIBC) recommended for T2-4a, N0-NxM0 MIBC, in the case of T1 bladder cancer not responsive to BCG treatment or not controllable by TURB[2]. In addition to the surgical skill required to perform RC, one of the challenges regarding this surgery is related to the patient’s medical condition. Surgery is generally performed in frail elderly patients, with several comorbidities, intractable gross hematuria and anemia, and some of them (about 10%-15%) are metastatic[2].
The term “salvage radical cystectomy” (SRC) initially referred to RC performed after bladder radiotherapy and implied an unfavorable meaning for the more elevated skill required to accomplish the procedure as well as its higher morbidity and mortality rate. Nowadays, SRC term is largely used when the bladder is removed in patients affected of MIBC who previously underwent unsuccessful initial trimodal treatment (TMT) or when RC is carried out for a purely palliative purpose aimed at treating only fatal disease-related complications and symptoms without a true oncologic intent.
We performed a literature review with the aim of summarizing the current role of salvage radical cystectomy in those two clinical settings of MIBC, after a failed initial treatment or as a palliative surgery.
METHODS
In January 2021, a literature research on PubMed/Medline, Scopus, and Google Scholar databases was performed by using the following keywords: bladder cancer, muscle invasive bladder cancer, bladder preservation, radiotherapy, pelvic irradiation, and salvage cystectomy. The title and the abstract of the retrieved studies were assessed for their relevance and, subsequently, their reference lists were screened to identify further studies. No time limit was applied to the research strategy, however English language restriction was used and no abstracts were included. In particular, two authors (Cicione A and Lombardo R) selected studies which included patients affected by MIBC who underwent salvage cystectomy as a subsequent treatment for supplementary control of disease and studies where RC was carried out only for a symptom-control purpose. Moreover, only studies using the Clavien-Dindo Classification System[3] were used to assess surgery complications of SRC.
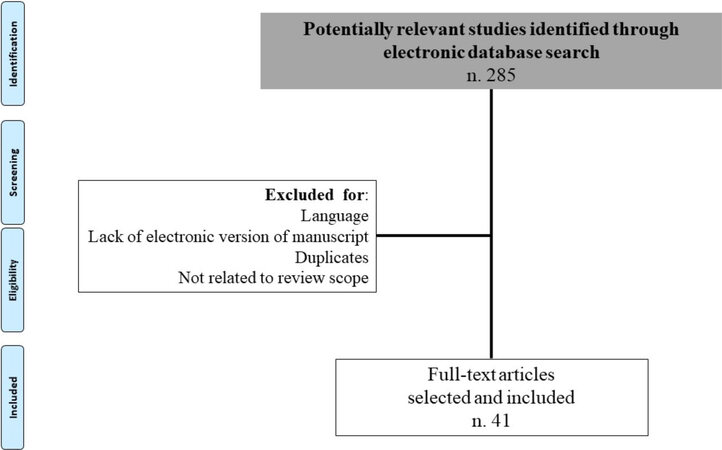
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines were respected in the preparation of this scoping review[4] [Figure 1].
RESULTS
Salvage radical cystectomy after trimodal therapy: oncological outcomes
Most of the retrieved studies reporting survival rates after SRC referred to cystectomy performed after preserving bladder treatment for MIBC [Table 1][5-18]. Moreover, there are no completed randomized trials comparing the oncological outcomes of preserving bladder treatment with RC[2], whereby the current oncological benefit of SRC after a bladder preserving treatment is based mainly on surgical series. At present, preserving bladder treatment is proposed to patients who refuse or are unfit for RC. The current clinical guidelines of the most popular oncological and urological societies [Table 2] suggest a bladder-preserving treatment known as “trimodality” or “trimodal treatment” to highly selected patients. The ideal patient is affected by an early stage MIBC without limpho-vascular invasion or distant metastases, in the absence of multifocal carcinoma in situ and hydronephrosis with a good bladder function and compliance for a long and close follow-up. The TMT protocol consists of preliminary extensive TURB aimed to remove all the visible tumor and allow a maximal focused radiation on the smallest tumor volume. Rödel et al.[19] showed that the extensiveness of TURB is the key component for any successful bladder-preservation strategy, and it was the only independent prognostic factor for long-term survival in their MIBCs series. Furthermore, the optimal radiation technique and dose have not yet been standardized, but two main protocols are used: split and continuous[20]. The first one includes bladder radiation with at least 40-45 Gy to the pelvis and concurrent radiosensitizing chemotherapy followed by an additional radiation boost to the bladder (20-25 Gy) if a complete response is documented on repeat biopsy[21]. The latter consists of full dose RT (64-66 Gy) with concurrent chemotherapy after maximal TURB[19]. Despite the controversies on the optimal RT protocols, the inferiority of RT alone compared to RT plus chemotherapy has been well established[2]. Thus, SRC in TMT setting is reserved for those patients who do not respond to treatment (immediate cystectomy) or develop an invasive recurrence during follow-up (delayed cystectomy).
Studies reporting survival outcomes after SRC following trimodal treatment for MIBC
| Ref. | Median age | Follow up | SRC patients (n) | Oncological outcomes |
| Eswara et al.[5] | 69.4 (27.5-88.9) | 12 years | 102 | Disease-free survival rates in the immediate and delayed groups was 38% and 61%, respectively, at 10 years (P < 0.05) with an overall 10-year disease-free survival rate of 48% for the entire cohort |
| George et al.[6] | - | 48.5 months | 11 | 7 patients died of disease, 2 died of other cause at 27 and 53 months, 1 was alive with distant metastases, and 1 was alive with no evidence of disease |
| Cooke et al.[11] | 65 (41-75) | 11 years | 38 | The median time to cystectomy after the primary treatment was 12 months (range 56 days to 10 years). The median survival after cystectomy was 15 months (95%CI: 9-23 months) |
| Nieuwenhuijzen et al.[12] | - | - | 27 | The 3- and 5-year survival probability after cystectomy was 46% (95%CI: 26-65) and 33% (11-54) |
| Bochner et al.[13] | - | - | 13 | After a median follow up of 28 months, 15 patients (82%) were without evidence of disease |
| Crawford et al.[14] | - | - | 34 | About 50% of patients died for disease progression with a mean survival time of 14.5 months |
| Freiha et al.[15] | - | - | 40 | 45% patients alive after 5 years |
| Swanson et al.[16] | 63 (41-79) | - | 62 | 14.5 months was the median time from the initial diagnosis of bladder cancer to cystectomy 5-year survival rate after cystectomy for the whole group was 43.2% |
| Abratt et al.[17] | 62 (36-82) | - | 46 | SRC was need after a mean of 11 months after radiotherapy. The overall 5-year survival rate was 43% while it was worse (7%) in case of higher grade and stage |
| Nurmi et al.[18] | 61 (32-74) | - | 20 | Intractable voiding symptoms were also reason for SRC. The overall 5-year survival rate after the operation was 61% |
| Linell et al.[7] | 66 (52-75) | - | 19 | SRC performed both for tumour recurrence and/or bladder symptoms The 5-year survival rate was 5 % |
| Konnak et al.[8] | 65 (50-82) | - | 18 | Interval between RT and SRC ranged between 6 months-12 years, mean 2.5 years The overall crude 5-year survival from the time of cystectomy was 50% |
| Smith et al.[9] | - | - | 80 | The over-all 5-year survival rate was 37% while the postoperative hospital mortality rate was 5% |
| Kulkarni et al.[10] | 71 (37-95) | 4.51 years | 6 | No significative difference in terms of 5-years DFS between radical cystectomy and TMT (respectively, 73.2% vs. 76.6%) was computed. SRC was performed in 6 (10.7%) of 56 patients who received TMT |
Current guidelines on bladder preserving approaches for MIBC
| Association | Patient selection criteria | Radiotherapy | Chemotherapy |
| AUA | Highly selected patient - Unfit for RC - Tumor resecable by TURB (< 3cm) - Absence of multifocal CIS and hydronefrosis and T3/T4 tumors - No histology variants - Well informed patient (40% subsequent RC) - Adequate bladder function - Follow-up | Halt the radiation at a dose of 40-45 Gy (approximately 2/3 of the total dose), repeat a cystoscopy with re-biopsy, and, if muscle invasive tumor still persists, recommend cystectomy at that time | Many prospective studies have reported high rates of local control (> 70%) in patients selected for treatment on protocols that included cisplatin with or without 5-FU |
| ESMO | Option for patients seeking an alternative to cystectomy and a palliative option for those who are medically unfit for surgery Ideal patient: early tumour stage (including high-risk T1 disease T2 < 5 cm), a visibly complete TURBT, absence of associated CIS and ureteral obstruction and adequate bladder capacity and function Lifelong surveillance is required to achieve optimal results | In case of bladder preservation with radiotherapy, combination with a radiosensitiser is always recommended to improve clinical outcomes, such as cisplatin, 5FU/MMC, carbogen/nicotinamide or gemcitabine | |
| EAU | Reasonable treatment option in well-selected patients as well as patients with a contraindication for surgery High level of patient compliance is need, absence of carcinoma in situ, absence or presence of hydronephrosis, optimal debulking of initial cancer | A standard radiation schedule includes EBRT to the bladder and limited pelvic LNs with an initial dose of 40 Gy, with a boost to the whole bladder of 54 Gy and a further tumour boost, with a total dose of 64 Gy | Different chemotherapy regimens have been used, but most evidence exists for cisplatin and mitomycin C plus 5-FU. In addition to these agents, other schedules have also been used, such as hypoxic cell sensitisation with nicotinamide, carbogen and gemcitabine, without clear preference for a specific radiosensitizer |
The first report on SRC was published in 1964 and reported discouraging results with three out of four patients dying due to sepsis. However, further series have been published over the years [Table 1]. One of the largest series included 159 patients initially affected by T2-T4 NxM0 MIBC that was managed by TMT[11]. All patients had a good performance status (EGOG score of 0-3) and cisplatin-based chemotherapy was administered. Over a mean follow-up of 11 years, 24% of patients required SRC after 12 months from initial treatment. After SRC, the median overall survival (OS) was 15 months. No significative difference in terms of survival was found for patients receiving SRC or not[11].
Eswara et al.[5] retrospectively analyzed clinical data of 348 patients undergoing TMT with extensive TURB, cisplatin-based chemotherapy, and almost 40 Gy radiotherapy. Patient’s features were similar to a previous study by Cooke et al.[11] except for the presence of hydronephrosis that was considered an exclusion criterion for trimodal treatment. On overage, SRC was carried out no more than 10.3 months after the last dose of chemotherapy. The 10-year disease free survival rate (DFS) from SRC was 48%, which significatively improved (up to 61%) when the surgery was delayed compared to conservative treatment.
Interestingly, Schuettfort et al.[22] recently used a pooled analysis method to assess the efficacy of trimodal treatment. They reviewed the available literature and identified 73 studies including 9110 patients. The analysis showed that SRC was necessary in 19.2% of cases and 5- and 10-year DFS rates were, respectively, 54.3% (95%CI: 48.6-60.1) and 45.6% (95%CI: 41.6-49.6).
Thus, 10%-30% of patients will require SRC after initial curative TMT with a mean 5-year DFS of 50% and 5-year OS rate of 33%-48%. However, those findings may be biased, hypothesizing that all patients requiring SRC were fit for surgery and studies included only patients with ≥ T2 N0M0 bladder cancer. In patients undergoing RC at primary MIBC diagnosis, Stein et al.[23] showed an OS at 5 and 10 years of 78% and 56%, respectively, in the presence of organ confined disease with no lymph node involvement. Those rates were dramatically reduced in the presence of extravesical disease extension (5-year OS = 55%; 10-year OS = 27%) and lymph nodes involvement (5-year OS = 31%; 10-year OS = 23%).
However, the rate of disease-free survival after SRC is higher than in the absence of treatment.
Overall, between 10% and 15% of patients are already metastatic at diagnosis with a median survival rarely exceeding 3-6 months before the development of effective chemotherapy[2]. Mak et al.[21] reported the rate of metastatic disease among 468 patients treated with TMT, which ranged from 32% to 35%. However, no studies on SRC with curative intent in this stage of disease were retrieved except for a palliative purpose.
Thus, we found that up to 30% of patients treated with primary TMT with a curative intent subsequently required SRC after 1 year of TMT. Although no comparative studies with RC are still available, SRC with a curative intent seems to be feasible with acceptable oncological outcomes. Moreover, the recent introduction of immunotherapy in the chemotherapeutic armamentarium encourages further assessing approaches that preserve the bladder.
Salvage radical cystectomy as palliative care: surgical outcomes
Bladder cancer is related to a significant morbidity for its debilitating symptoms. Among them, hematuria is the most common presenting symptom occurring in approximately 85% of diagnosed cases. Beyond the tumor mass, side effects of radiation or upper urinary tract neoplasms may be further reasons for bleeding. Sometimes hematuria may be difficult to control, uncurable by irrigation or hemostatic trans-urethral resection, and thus is potentially life threatening. Furthermore, the recurrence of gross intractable hematuria is a significant concern, worsening the quality of residual life. Thus, SRC with a palliative intent may be an effective treatment of choice.
Zebic et al.[25] carried out seven SRCs with a palliative intent, namely the surgical indication was due to T4a bladder cancer (3 patients) and pelvic malignancies leading to severe voiding symptoms, pain, and hematuria with need for repeated blood transfusions. Among them, 3 patients were lost during follow-up, 2 patients died during recovery for complications, and 2 patients lived 366 days after surgery. The preoperative risk was assessed by ASA score, resulting 4 patients ASA 4, two ASA 2, and one ASA 1.
Nagele et al.[26] investigated clinical outcomes of 20 patients, with a mean age of 64 years, undergoing SRC for T4-stage bladder cancer. After a mean follow-up of 13 months (range 1-36 months), 11 patients were still alive. The authors reported only one lethal complication, namely an enterocutaneous fistula occurring during recovery. No data on preoperative surgical risk were reported.
The study of Cochetti et al.[27] included 12 patients who underwent RC for massive hematuria due to bladder cancer and causing severe anemia (Hb level < 8 g/dL). The pathological exam showed pT4 stage in 6 patients, 2 patients with pT2, and 4 patients affected by pT3 stage disease. Major complications occurred in 18.5% of cases, while no deaths were recorded. Although all patients were defined as ASA 4, the mean Charlson Index was 6 and median Karnofsky scale was 85. An ileal conduit was used as a urinary diversion in all studies mentioned above, while ureterocutaneostomy was performed in the presence of severe comorbidities and poor performance status.
Thus, the studies all showed that, if technically feasible, in patients with a decent frailty status and accepting surgery, the future problems of bleeding may be completely obviated. Frailty is a new concept introduced to estimate the patient’s vulnerability to stress factors such as surgery. It has been recently developed in the context of bladder cancer because of the patient’s median age at diagnosis, which makes the presence of several comorbidities highly probable[28]. Although a variety of methods are available to measure frailty, a poor score has usually been associated with worse postoperative outcomes in patients who undergo urologic surgery including RC[28].
When SRC is not possible due to the patient’s elevated frailty status or the patient’s refusal, several alternatives to a radical treatment have been proposed.
Since 1960, low-dose RT has been adopted to control hematuria. Regarding treatment outcomes, at 2 weeks, the rate of efficacy in arresting bladder bleedings has been reported as 60%-69%, while the risk of recurrence at 6 months has been computed as 33%-69%[29-31].
Selective angiography for bladder embolization showed a high success rate (82%-100%) with complete cessation of hematuria within 48 h and a bleeding recurrence risk of 28%-50% within 16 months[32]. However, this option is not free from complications. Ischemic pain, bladder necrosis, bladder infarction, and even inadvertent occlusion of uninvolved vessels by refluxed embolic material have been reported[33].
Finally, several endovesical agents have been used to stop bladder bleeding such as 1% silver nitrate or 1%-2% alum and formalin (2.5%-4% for 30 min) with response rates of 71%-100% and 5%-100%, respectively. However, the treatment lasted 1-5 days, and in all cases anesthesia was required.
Salvage radical cystectomy: morbidity and mortality
SRC is thought to be difficult because of previous radiotherapy. Pelvic RT results in tissue damage that can also affect surrounding organs and lead to desmoplastic reaction, obliterating the tissue plane. This makes it difficult to identify and dissect surgical structures[34].
By researching studies only using Clavien-Dindo system to grade complications, we found three single-institution studies[5,35,36] which assessed complications of SRC after RT for MIBC and one multicenter study[37] where SRC was carried out after RT for further diseases [Tables 3 and 4].
Studies on complications after salvage radical cystectomy
| Ref. | N of patients undergone to SRC | Findings |
| Iwai et al.[35] | 87 | 40 Gy administered. Retrospective in nature comparing 87 SRC vs. 106 RC Urinary anastomosis-related complications and major gastrointestinal complications, most of which were Grade 3 ileus, were more frequent in the SRC respectively: 11% vs. 2%, P = 0.007 and 14% vs. 4%, P = 0.002 |
| Eswara et al.[5] | 91 | Induction RT dose 40 Gy + 25 Gy consolidation in case of positive initial response Major complications, CCS ≥ 3, occurred in 15 patients (16%). The overall 90-day complication rate was 69%. Perioperative mortality rate within 90 days was 2.2% |
| Eisenberg et al.[36] | 148 | Radiotherapy by 70 Gy. 90-day overall complication rate was 77%. Among them, 44.6% were low grade and 32.4% high-grade. The type of urinary diversion was not related to complication occurence |
| Gontero et al.[37] | 682 | Retrospective in nature from SRCs carried out in 25 high volume centers (more than 30 procedures per year). Overall rate of complications was 75.1%; CCS ≥ 3 in 29.6% and CCS = 5 in 2.9% of patients. 27% of patients received RT for bladder cancer. Mean RT dose was 63 Gy (51-70) |
Reported range of complications graded by Clavien-Dindo System
| Grade ≥ 3 | Grade < 3 | |
| Infection (wound, urinary tract, others) | 3-7 | 4-43 |
| Gastrointestinal (ileus, bowel perforation) | 8-14 | 10-17 |
| Urinary anastomosis-related (leakage, stricture) | 2-5 | 3-7 |
Gontero et al.[37] retrospectively analyzed data from 25 large-volume institutions, defined as more than 30 cystectomies per year. Although only 27% of patients previously received RT for bladder cancer, the authors showed that the SRC is associated with a high risk of morbidity (75% risk of a single complication and a 33% risk of a major complication) and a 3.1% mortality rate at 90 days after surgery. Only large-volume centers participated in this study. Surgeon volume had a greater impact on outcomes in RC when compared with other surgeries such as lung resection for cancer, abdominal aortic aneurysm repair, and coronary artery bypass[38]. Furthermore, it has been computed that more than 20 RCs per year positively affected the complication rates when compared to a lower number[39].
Iwai et al.[35] compared complication rates in patients with and without previous TMT using the standardized Clavien-Dindo grade. Data analysis showed that previous chemoradiotherapy increases the risk of urinary anastomosis-related complications (such as stricture and urinary leakage) and is associated with gastrointestinal complications (such as bowel perforation and Grade 3 ileus).
According to the authors, these complications would result, at least in part, from compromised blood supply to the tissue because of previous RT. Most patients (84%) received an ileal conduit as a urinary diversion, while the others received orthotopic ileal neobladder (6%), Indiana pouch (3%), or ureterocutaneostomy (7%). When univariate analysis was carried out to identify risk factors associated with urinary and bowel complications, the type of urinary diversions was not a predictor.
Eisenberg et al.[36] reviewed clinical data of RCs performed in their tertiary referral care center. In 148 patients who underwent SRC, they computed a 32.4% rate of high-grade complications (CCS ≥ 3). Again, ileal conduit was the most used urinary diversion (43.9%), and this was not related to the occurrence of complications, while ASA score and patients age were predictors.
Finally, in the study by Eswara et al.[5], which included 192 SRCs, major complications, Grades 3-5, occurred in 15 patients (16%) for a total of 23 events. The perioperative mortality rate within 90 days was 2.2%. Ileal conduit was the only used urinary diversion. However, the main finding of their study was to stratify complications occurrence by the date of SRC. Although there were no significant differences in the number of total complications, tissue healing-related complications occurred nearly three times more frequently (35% vs. 12%, P = 0.05) in the case of late SRC, namely disease recurrence after a mean of 10.3 months (range 2.1-178 months) from TMT termination. This group of complications included wound infection, ureteral stricture, anastomotic stricture, and stoma/loop requiring revision. Again, the authors explained this finding by assuming the higher dose (mean 64.7 Gy vs. 39.9 Gy) of radiation received.
All these studies reported occurrence of urinary anastomosis-related complications and major gastrointestinal complications more likely in the case of a previous radiotherapy that presumably caused an endarteritis process with subsequent ischemia delaying wound healing[40].
CONCLUSION
Salvage radical cystectomy is performed both after failure of conservative treatment for muscle invasive bladder cancer and as a palliative surgery in the case of intractable and fatal complications such as hematuria. An appropriate selection of patients suited for TMT leads to acceptable outcomes, whereas the rate of major complications (CCS ≥ 3) in the case of a subsequent SRC is higher than the one previously reported for RC[41]. Furthermore, during patient counseling for TMT, the high probability of receiving an incontinent urinary diversion in the future instead of an orthotopic neobladder in the case of immediate RC should be underlined. In the absence of comparative studies able to identify risk factors for TMT failure, a multidisciplinary cooperation and close follow-up is required. Likewise, SRC for symptom relief should be considered only if there are no other options and after an accurate assessment of patient frailty through the currently available questionnaires such as ASA score Charlson Index, Karnofsky scale, and Geriatric-8 currently able to estimate patient’s vulnerability to stress factors such as surgery.
DECLARATIONS
Authors’ contributionsData research and manuscript drafting: Cicione A, Lombardo R, Voglino OA
Manuscript revision: Tubaro A, De Nunzio C
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Cicione A, De Nunzio C, Lombardo R, et al. Complications and quality of life of ileal conduit, orthotopic neobladder and ureterocutaneostomy: systematic review of reports using the Clavien-Dindo Classification. Minerva Urol Nefrol 2020;72:408-19.
2. Alfred WJ, Lebret T, Comperat EM, et al. Updated 2016 EAU Guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 2017;71:462-75.
3. Mitropoulos D, Artibani W, Graefen M, et al. Reporting and grading of complications after urologic surgical procedures: An ad hoc EAU Guidelines Panel Assessment and Recommendations. Eur Urol 2012;61:341-9.
4. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535.
5. Eswara JR, Efstathiou JA, Heney NM, et al. Complications and long-term results of salvage cystectomy after failed bladder sparing therapy for muscle invasive bladder cancer. J Urol 2012;187:463-8.
6. George L, Bladou F, Bardou VJ, et al. Clinical outcome in patients with locally advanced bladder carcinoma treated with conservative multimodality therapy. Urology 2004;64:488-93.
8. Konnak JW, Barton Grossman H. Salvage cystectomy following failed definitive radiation therapy for transitional cell carcinoma of bladder. Urology 1985;26:550-3.
9. Smith JA, Whitmore WF. Salvage cystectomy for bladder cancer after failure of definitive irradiation. J Urol 1981;125:643-5.
10. Kulkarni GS, Hermanns T, Wei Y, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol 2017;35:2299-305.
11. Cooke PW, Dunn JA, Latief T, Bathers S, James ND, Wallace DMA. Long-term risk of salvage cystectomy after radiotherapy for muscle-invasive bladder cancer. Eur Urol 2000;38:279-86.
12. Nieuwenhuijzen JA, Horenblas S, Meinhardt W, Van Tinteren H, Moonen LMF. Salvage cystectomy after failure of interstitial radiotherapy and external beam radiotherapy for bladder cancer. BJU Int 2004;94:793-7.
13. Bochner BH, Figueroa AJ, Skinner EC, et al. Salvage radical cystoprostatectomy and orthotopic urinary diversion following radiation failure. J Urol 1998;160:29-33.
16. Swanson DA, von Eschenbach AC, Bracken RB, Johnson DE. Salvage cystectomy for bladder carcinoma. Cancer 1981;47:2275-9.
17. Abratt RP, Wilson JA, Pontin AR, Barnes RD. Salvage cystectomy after radical irradiation for bladder cancer-prognostic factors and complications. Br J Urol 1993;72:756-60.
18. Nurmi M, Valavaara R, Puntala P, Ekfors T. Single-stage salvage cystectomy: results and complications in 20 patients. Eur Urol 1989;16:89-91.
19. Rödel C, Grabenbauer GG, Kühn R, et al. Organ preservation in patients with invasive bladder cancer: Initial results of an intensified protocol of transurethral surgery and radiation therapy plus concurrent cisplatin and 5-fluorouracil. Int J Radiat Oncol Biol Phys 2002;52:1303-9.
20. Polo-Alonso E, Kuk C, Guruli G, et al. Trimodal therapy in muscle invasive bladder cancer management. Minerva Urol Nefrol 2020;72:650-62.
21. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of radiation therapy oncology group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801-9.
22. Schuettfort VM, Pradere B, Quhal F, et al. Incidence and outcome of salvage cystectomy after bladder sparing therapy for muscle invasive bladder cancer: a systematic review and meta-analysis. World J Urol 2020; doi: 10.1007/s00345-020-03436-0.
23. Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19:666-75.
24. Martini A, Sfakianos JP, Renström-Koskela L, et al. The natural history of untreated muscle-invasive bladder cancer. BJU Int 2020;125:270-5.
25. Zebic N, Weinknecht S, Kroepfl D. Radical cystectomy in patients aged ≥ 75 years: an updated review of patients treated with curative and palliative intent. BJU Int 2005;95:1211-4.
26. Nagele U, Anastasiadis AG, Merseburger AS, et al. The rationale for radical cystectomy as primary therapy for T4 bladder cancer. World J Urol 2007;25:401-5.
27. Cochetti G, Barillaro F, Boni A, Mearini E. Immediate radical cystectomy for massive bleeding of bladder cancer. Biomed Res Int 2015;2015:154392.
28. De Nunzio C, Cicione A, Izquierdo L, et al. Multicenter analysis of postoperative complications in octogenarians after radical cystectomy and ureterocutaneostomy: the role of the frailty index. Clin Genitourin Cancer 2019;17:402-7.
29. Lacarrière E, Smaali C, Benyoucef A, Pfister C, Grise P. The efficacy of hemostatic radiotherapy for bladder cancer-related hematuria in patients unfit for surgery. Int Braz J Urol 2013;39:808-16.
30. Wujanto C, Tey J, Chia D, et al. Radical radiotherapy in older patients with muscle invasive bladder cancer. J Geriatr Oncol 2019;10:292-7.
31. Abt D, Bywater M, Engeler DS, Schmid HP. Therapeutic options for intractable hematuria in advanced bladder cancer. Int J Urol 2013;20:651-60.
32. Loffroy R, Pottecher P, Cherblanc V, et al. Current role of transcatheter arterial embolization for bladder and prostate hemorrhage. Diagn Interv Imaging 2014;95:1027-34.
33. Mohan S, Kumar S, Dubey D, Phadke RV, Baijal SS, Kathuria M. Superselective vesical artery embolization in the management of intractable hematuria secondary to hemorrhagic cystitis. World J Urol 2019;37:2175-82.
34. Ramani VAC, Maddineni SB, Grey BR, Clarke NW. Differential complication rates following radical cystectomy in the irradiated and nonirradiated pelvis. Eur Urol 2010;57:1058-63.
35. Iwai A, Koga F, Fujii Y, et al. Perioperative complications of radical cystectomy after induction chemoradiotherapy in bladder-sparing protocol against muscle-invasive bladder cancer: a single institutional retrospective comparative study with primary radical cystectomy. Jpn J Clin Oncol 2011;41:1373-9.
36. Eisenberg MS, Dorin RP, Bartsch G, Cai J, Miranda G, Skinner EC. Early complications of cystectomy after high dose pelvic radiation. J Urol 2010;184:2264-9.
37. Gontero P, Pisano F, Palou J, et al. Complication rate after cystectomy following pelvic radiotherapy: an international, multicenter, retrospective series of 682 cases. World J Urol 2020;38:1959-68.
38. Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117-27.
39. Nielsen ME, Mallin K, Weaver MA, et al. Association of hospital volume with conditional 90-day mortality after cystectomy: an analysis of the National Cancer Data Base. BJU Int 2014;114:46-55.
40. Mak RH, Zietman AL, Heney NM, Kaufman DS, Shipley WU. Bladder preservation: Optimizing radiotherapy and integrated treatment strategies. BJU Int 2008;102:1345-53.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Cicione A, Lombardo R, Voglino OA, Tubaro A, De Nunzio C. An investigative review on the current role and outcomes of salvage radical cystectomy. Mini-invasive Surg 2021;5:47. http://dx.doi.org/10.20517/2574-1225.2021.52
AMA Style
Cicione A, Lombardo R, Voglino OA, Tubaro A, De Nunzio C. An investigative review on the current role and outcomes of salvage radical cystectomy. Mini-invasive Surgery. 2021; 5: 47. http://dx.doi.org/10.20517/2574-1225.2021.52
Chicago/Turabian Style
Cicione, Antonio, Riccardo Lombardo, Olivia Alessandra Voglino, Andrea Tubaro, Cosimo De Nunzio. 2021. "An investigative review on the current role and outcomes of salvage radical cystectomy" Mini-invasive Surgery. 5: 47. http://dx.doi.org/10.20517/2574-1225.2021.52
ACS Style
Cicione, A.; Lombardo R.; Voglino OA.; Tubaro A.; De Nunzio C. An investigative review on the current role and outcomes of salvage radical cystectomy. Mini-invasive. Surg. 2021, 5, 47. http://dx.doi.org/10.20517/2574-1225.2021.52
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 5 clicks
Cite This Article 5 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.