Prevention and management of peri-procedural TAVR complications
Abstract
Transcatheter aortic valve replacement (TAVR) is a safe and effective treatment strategy for severe aortic stenosis. However, peri-procedural complications can have a significant impact on acute and longer-term morbidity and mortality. Therefore, this review article provides a practical overview on how to prevent and manage the common and also rare but life-threatening peri-procedural TAVR complications.
Keywords
INTRODUCTION
Transcatheter aortic valve replacement (TAVR) is a recommended treatment strategy for patients with severe aortic stenosis (AS) across all surgical risk profiles. As TAVR expands towards lower-risk younger patients, the number of procedures is set to dramatically increase. Knowledge of how to prevent and manage peri-procedural complications, which can be life-threatening or have a significant impact upon long-term morbidity and mortality, is therefore crucial. This review aims to provide interventionists with a practical guide for the prevention and management of important peri-procedural complications during TAVR.
VASCULAR COMPLICATIONS
Vascular complications encompass a wide spectrum of conditions, including damage to aortic and ventricular structures or peripheral access site-related injuries, with the latter being the focus of this section[1]. Development of a vascular complication is associated with increased morbidity, mortality, and length of hospital stay and worsening quality of life[2,3]. Despite improvements in operator experience, pre-procedural planning, procedural access and closure techniques, and dramatic reductions in valve profiles, the rate of major vascular complications in contemporary cohorts still ranges 5%-10%[4-7]. Furthermore, the fact that TAVR is inferior to surgical aortic valve replacement (SAVR) in terms of vascular complications will become increasingly relevant as TAVR expands to lower-risk younger patients[4]. Therefore, this section focuses on the practical pre-procedural and intra-procedural steps that should be considered in order to reduce the incidence and impact of any vascular complications.
Preventing vascular complications
A detailed evaluation of the peripheral vessels using pre-procedural multi-slice computed tomography (MSCT) is critical to reducing the risk of vascular complications. The following variables should be examined: minimal lumen diameters of the iliac and femoral vessels (> 5.5 mm), ilio-femoral vessel tortuosity, vessel calcification location, length and arc, location of femoral bifurcation, and presence of any additional vascular pathology[8-10]. When feasible, trans-radial access can be considered for the contralateral diagnostic approach and is associated with a significant reduction in vascular complications compared to a conventional bi-femoral approach[11,12].
In the presence of severe, ilio-femoral calcific stenosis, peripheral intravascular lithotripsy (IVL) may facilitate trans-femoral access. Among 42 patients with unfavorable ilio-femoral anatomy, use of IVL facilitated a successful trans-femoral procedure in 90% of cases, with only two patients developing vascular complications (pseudoaneurysm and requirement for endarterectomy)[13]. If, however, both ilio-femoral access routes are not feasible, then an MSCT evaluation of alternative access sites should be undertaken. The most frequent non-transfemoral approaches include trans-carotid and trans-axillary approaches[14-16]. Data from the FRANCE transcatheter aortic valve implantation (TAVI) registry show that the use of trans-axillary and trans-carotid access was associated with similar rates of mortality and access site complications and a lower rate of major vascular complications and unplanned vascular repairs compared to transfemoral access[15]. In patients with no peripheral access option available, trans-caval access can be considered and initial experience is promising with procedural and device success occurring in 99/100 patients[17,18]. Rates of Valve Academic Research Consortium (VARC)-2 bleeding and major vascular complications were 7% and 13% in this population cohort. Finally, if no percutaneous solutions are possible, then direct surgical access can be considered, as either a cut-down approach to the axillary or carotid arteries or a more traditional aortic access via mini-thoracotomy.
Access site management
Using the pre-procedural computed tomography (CT), the optimal site for arterial puncture can be determined. Use of real-time ultrasound guidance is increasing and is associated with a reduction in the incidence of vascular complications[19]. Ultrasound can assist in localization of the femoral bifurcation and presence of anterior wall calcification. Adjunctive techniques include “road-mapping” the femoral puncture site by performing a diagnostic angiogram from the contralateral site. Adequate preparation of the sub-cutaneous tract overlying the arterial puncture site can aid in the advancement of progressively larger sheaths and vascular closure devices (VCD).
TAVR delivery sheath technology has now evolved whereby most contemporary valves can now be delivered through 14 Fr sheaths. Modern delivery sheaths such as the iSLEEVE (Boston Scientific), eSheath (Edwards Lifesciences), and Python (Meril Lifescienes) consist of an inner folded membrane in the proximal portion which can expand up to 18 Fr inside the descending aorta to accommodate delivery of the subsequent valve[20].
At the end of the procedure, closure of large arteriotomies can be safely and effectively achieved using VCDs. Their use is associated with a reduction in procedural time, hospital stay, and complication rates[21,22]. However, VCD failure is still the leading cause of major vascular complications. Multiple suture-based and collage plug-based solutions exist on the market. For larger arteriotomies, pre-closure with two parallel ProGlides or one Prostar XL is often preferred[23,24]. Head-to-head comparisons of the two suture-based approaches has yielded conflicting results[21,22,24-26], but in both cases choice of arterial puncture and preparation of the sub-cutaneous tract are essential to ensuring a successful outcomes.
An alternative is the MANTA device (Teleflex Inc, PA, USA), which is the only commercially available VCD for large bore arterial access[27]. It comes in two sizes, the 14 Fr and 18 Fr MANTA, which are indicated for closure of 10-14 and 15-20 Fr sheaths/devices, respectively. Technical success ranges 96%-98% with a rate of vascular complications of 2%-5%, requiring percutaneous or surgical intervention in around 1%-2% due to vessel dissection or stenosis/occlusion[27-30]. The recent MASH (MANTA vs. Suture-based vascular closure after transcatheter aortic valve replacement) randomized trial found no difference between the two strategies in terms of access site-related vascular complications or clinically relevant bleeding[31]. However, the use of ProGlide was associated with more device failures, whilst MANTA required greater use of covered stents/surgical bailouts.
Once the delivery sheath is removed, a final check angiogram of the main access site should be performed to assess for vessel dissection or perforations. Routine placement of a crossover wire from the contralateral diagnostic access site is recommended to enable prompt treatment of any complications. In the majority of cases, occlusive balloon inflation is sufficient to resolve minor perforations or vessel dissections. In cases with prolonged bleeding, covered stenting might be considered, and, if there is extensive vascular injury, persistent flow-limiting dissection, or acute limb-threatening ischemia, then surgical repair is indicated.
ANNULAR RUPTURE
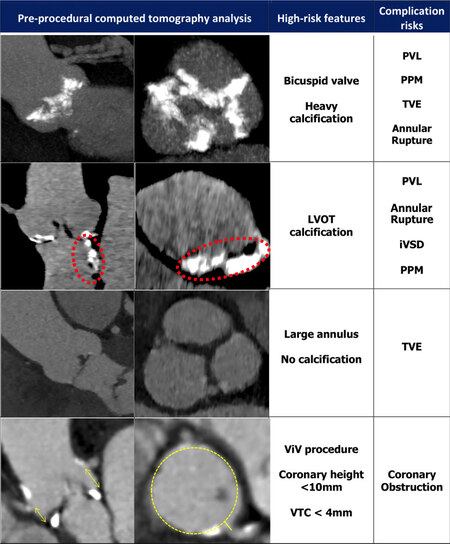
Annular rupture remains one of the most feared complications of TAVR due to the potential rapid onset of hemodynamic collapse and high rate of mortality. It encompasses injuries that can occur to the aortic annulus, aortic sinuses, and root and left ventricular outflow tract (LVOT), collectively referred to as the “device landing zone” (DLZ)[32,33]. Its reported incidence is < 1% and usually manifests itself acutely intra-procedurally, although delayed presentations have been described[34]. Risk factors for annular rupture include heavy annular or DLZ calcifications, shallow sinuses of Valsalva, small aortic annulus (< 20 mm), device over-sizing (> 20%), bicuspid aortic valve, and severe asymmetric sub-annular left ventricle (LV) hypertrophy in the presence of LVOT calcification [Figure 1][32,35,36]. Therefore, detailed multi-slice CT (MSCT) analysis of the DLZ and aortic sinus geometry is critical to minimizing the risk of annular rupture[35,37].
Figure 1. High-risk multi-slice computed tomography (MSCT) features for TAVR complications. Examples of anatomical phenotypes that can predispose the patient to the development of specific peri-procedural complications. PVL: Paravalvular leak; ViV: valve-in-valve; LVOT: left ventricular outflow tract; PPM: permanent pacemaker; TVE: transcatheter valve embolization; iVSD: iatrogenic ventricular septal defect; VTC: virtual transcatheter-to-coronary distance.
Attention should be given to the overall extent, distribution, and type of calcification[37,38]. In high-risk anatomies, a self-expandable valve (SEV) is preferred with caution advised for post-dilatation, or, if a balloon-expandable valve (BEV) is necessary, then a degree of under-filling may be required. For highly eccentric annuli, perimeter-based sizing can be used. In cases with severe LVOT calcification, a higher implantation can be considered to reduce the radial force of the valve upon the vulnerable LVOT. Given that valve over-sizing (> 20%) is a risk factor for aortic rupture, accurate annular sizing is critical. In cases where MSCT measurements are challenging or not possible, balloon sizing can be performed with aortography used to confirm the absence of lateral aortic regurgitation (AR) with fully inflated balloons of known sizes. An alternative non-invasive approach is to use 3D transesophageal echocardiography to evaluate annular dimensions.
Management of annular rupture
The clinical presentation and subsequent treatment depend on the location and extent of the annular rupture. In the extreme, it can present suddenly with hemodynamic collapse resistant to inotropic support often with pericardial tamponade. Immediate aortography and echocardiography can confirm the diagnosis and rule out other differentials of acute hypotension such as cardiac perforation, coronary occlusion, or major vascular bleeding. For large un-contained ruptures, cardio-pulmonary bypass should be established immediately with subsequent median sternotomy for surgical exploration and repair. Use of extracorporeal membrane oxygenation (ECMO) is not helpful and may delay definite surgical management.
In exceptional cases, due to the extreme surgical risk of these patients, percutaneous bail-out measures have been described. The annular tear can be sealed using vascular plugs, embolization coils, implantation of a vascular occlusion device in cases of muscular ventricular septal defect perforations, and less successfully implantation of a second transcatheter heart valve (THV)[39-41,42]. In our experience, reversal of heparin can be resolutive when extravasation is limited. For more limited or contained ruptures, a conservative approach can be considered with reversal of heparin, prompt pericardial drainage, and close repeated echocardiographic or MSCT surveillance[43]. Echocardiography is also useful to detect subtle signs of aortic injury such as presence of effusion in the transverse sinus, subepicardial hematoma at the base of the heart, peri-aortic hematoma, new aortic wall thickening, and local or extended aortic dissection[32,44].
AORTIC AND VENTRICULAR
Iatrogenic aortic dissection is more common in heavily diseased, calcified, and tortuous aortic anatomies[45]. Care should be taken during device and sheath manipulation in these aortas with wire and catheter exchanges minimized. In a tortuous or horizontal aorta, an additional stiff wire advanced into the aortic root can enhance the support and deliverability of the THV. In cases of extremely hostile aortas, trans-apical TAVR can be considered as an alternative access route.
Improvements in device technology and operator experience has reduced the incidence of cardiac tamponade down to around 1%[46]. Right ventricular perforation is more common and can occur due to placement of the temporary pacing wire. Use of a balloon-tipped pacing wire or pacing via the LV guidewire can therefore lower this risk[47]. Isolated right ventricular perforation rarely requires surgery and usually conservative management with pericardiocentesis is sufficient. In contrast, LV perforation almost always requires prompt surgical repair. The use of softer pre-shaped LV guiding wires has reduced the incidence of this complication.
TRANSCATHETER VALVE EMBOLIZATION
Transcatheter valve embolization (TVE) is defined as movement of valve prosthesis after deployment such that it loses contact with the annulus. It has a reported incidence of around 1% and is associated with elevated post-procedural morbidity and mortality and need for conversion to open heart surgery[48-51]. TVE can occur in the aortic or ventricular direction and usually occurs during the peri-procedural period, however delayed migration (> 24 h post-procedure) has been reported[49,52,53].
Minimizing risk of transcatheter valve embolization
Multiple anatomical, procedural, and THV risk factors for TVE have been identified[49]. On pre-procedural MSCT, attention should be given to the presence of significant aortic tortuosity, excessively small or large annuli, complete absence or presence of heavy calcification, and LVOT hypertrophy [Figure 1]. Pure aortic regurgitation is usually associated with the combination of large annuli, absence of significant calcification for device anchoring, and larger stroke volume, all of which can increase the risk for TVE. To mitigate this risk, device over-sizing and rapid ventricular pacing during deployment is recommended. Additionally, dedicated THV such as the Jena valve (Jena Valve Technologies, Irvine CA) or J-valve (JC Medical, Burlingame, CA) can be considered. When treating bicuspid aortic valve stenosis with heavy calcification, a THV with increased radial strength should be selected to avoid device under-expansion and subsequent risk of TVE.
Intra-procedurally, failure of rapid ventricular pacing during valve deployment can lead to a sudden subsequent migration or embolization of the THV[54]. Therefore, stability of the pacing system should be confirmed prior to valve crossing and deployment. Following valve deployment, caution should be taken during retraction of the device nose-cone as well as the jailed pigtail catheter, which should be removed with the aid of a 0.035" wire.
Management of transcatheter valve embolization
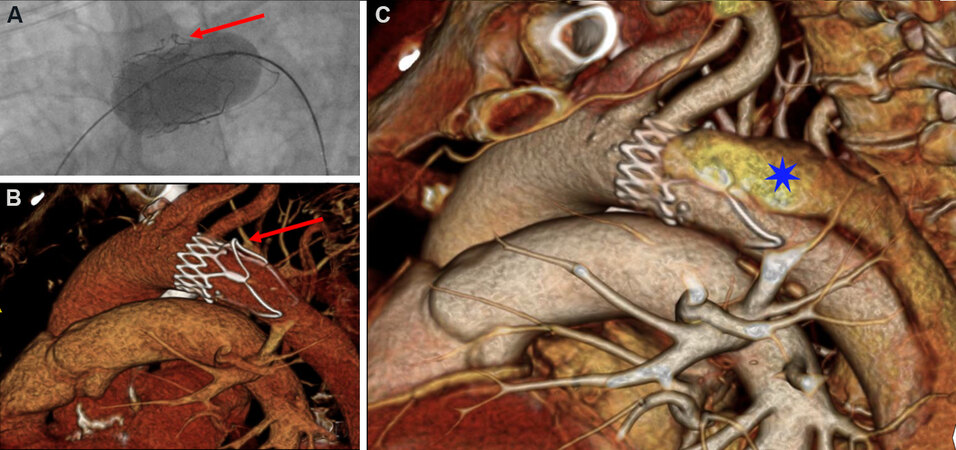
Deep ventricular embolization nearly always requires open surgical repair. Implanting a second THV to trap the embolized valve prior to surgical conversion may limit the duration and complexity of the subsequent surgical intervention[55]. In contrast, aortic embolizations can be managed interventionally, with surgery reserved for cases complicated by aortic injuries such as dissections or perforations [Figure 2]. If the extent of aortic embolization is minimal, the first THV can be fixed by a second THV implanted in the appropriate location[56].
Figure 2. Transcatheter valve embolization complicated by aortic dissection. (A) Aortic embolization of an ACURATE neo transcatheter valve was managed by using an inflated balloon to retract the device into the aortic arch. (B) The re-positioning maneuver caused an infolding of one of the upper stabilizing arches of the valve frame (red arrow). (C) This resulted in a type B aortic dissection (blue star), which was conservatively managed.
In more extensive embolization, repositioning maneuvers using snares or an inflated valvuloplasty balloon can be used to grasp, move, and deploy the embolized valve at an ectopic location[57]. If embolization is complicated with coronary obstruction, then immediate proximal retraction of the device should be performed. Care must be taken while manipulating the THV to avoid aortic injury and dissections, especially with certain SEV which have more prominent outflow portions [Figure 2]. During repositioning of an embolized BEV, the ventricular guidewire should always be maintained within the valve to prevent inversion of the valve and potential flow obstruction. The preferred location for deployment is in the descending aorta, distal to the left subclavian artery taking care to avoid any major aortic branches. However, in tortuous, heavily diseased or calcified aortas where the risk of aortic injury during re-positioning maneuvers is high, deployment at a more proximal position can be considered and was found to not be associated with an elevated stroke rate[49]. Implantation of a covered or uncovered stent within the embolized valve leaflets can be undertaken to fixate the valve against the aortic wall. Mid-term follow-up of ectopically implanted THVs suggests there is a minimal risk for further migration or subsequent vascular functional[49,56,58].
CORONARY OBSTRUCTION
The incidence of coronary obstruction is < 1% for native valve TAVR and increases up to 3.5% for valve-in-valve (ViV-TAVR)[59-61]. It presents acutely intra-procedurally with hypotension, ischemic electrocardiogram (ECG) changes, and usually involves the left coronary ostium[59]. Delayed coronary obstruction can occur up to one-year post TAVR and is more commonly seen following ViV procedures or with use of a SEV[62].
Strategies for high-risk coronary obstruction
High-risk anatomical features for coronary obstruction (CO) include low coronary ostia (< 10 mm) and the presence of narrow shallow aortic sinuses[59]. For ViV-TAVR, additional factors include the presence of a stentless or stented valve with externally mounted leaflets and a virtual transcatheter distance of less than
In patients found to be at high risk for CO, SAVR should be considered. If not feasible due to the operative risk of the patient, then multiple strategies can be considered. Use of a partially or fully recapturable THV (Evolut-R/PRO, Portico, LOTUS) or a THV with a favorable open-celled design (ACURATE neo, NAVITOR) may be advantageous. Newer devices with unique leaflet grasping mechanisms (Jena and Engager) may theoretically be advantageous in a ViV setting. Techniques to achieve commissural alignment with the Evolut and ACURATE neo platforms have been described and can successfully reduce the incidence of severe coronary overlap[64-67]. However, the impact of commissural alignment on the subsequent risk for coronary obstruction remains to be evaluated.
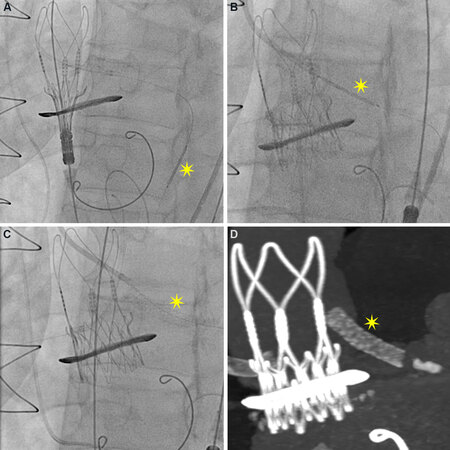
Prior to valve deployment, prophylactic placement of a coronary guidewire with or without an undeployed stent can be considered[68,69]. Following valve deployment, if coronary obstruction ensues or coronary flow is reduced, then the stent can be retracted and implanted halfway between the proximal coronary artery and THV as per the “chimney” or “snorkel” technique [Figure 3][70]. To avoid stent under-expansion or deformation, a stent with a high radial strength should be used followed by high-pressure post-dilatation, with intravascular ultrasound (IVUS) used to evaluate for adequate stent expansion. If post-dilatation of the THV is required, then a simultaneous kissing balloon inflation of the THV and coronary stent can be performed. During ViV-TAVI, angiographic assessment may not be sufficient to decide whether stent implantation is required. In this setting, IVUS can be used to guide decision making by identifying specific markers of CO[71].
Figure 3. Coronary protection with “chimney stenting” technique. Valve-in-valve procedure for a patient at high risk for coronary obstruction (low-lying coronary ostia and narrow sinuses). (A) Left coronary ostium was engaged and a wire and stent (yellow star) placed distally in the artery prior to valve deployment. (B) Following valve deployment, the stent was retracted and (C) implanted adjacent to the transcatheter valve. (D) The CT reconstruction image shows half the stent placed in the ostium and the other half protruding into the aorta.
When utilized, the chimney technique is associated with good mid-term follow-up and a low incidence of stent thrombosis of < 1%[68,69]. Given that a portion of the stent protrudes into the aorta and is unlikely to undergo reendothelialization, prolonged dual anti-platelet therapy (DAPT) therapy should be considered.
An alternative to chimney stenting is the bioprosthetic aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) technique to split either native or bioprosthetic aortic valve leaflets prior to TAVR implantation in order to maintain blood flow into the coronary sinus[72,73]. The procedure involves intentional laceration of the leaflets using radiofrequency energy delivered to a guidewire suspended between two guiding catheters[74]. The procedure requires specific devices and equipment with in-depth pre-procedural MSCT analysis to identify the optimal candidates for BASILICA[73,75-77]. The recently reported results of 214 patients from the BASILICA registry are promising with procedural success, defined as successful BASILICA traversal and leaflet laceration without mortality, coronary obstruction, or emergency intervention, achieved in 86.9% of patients[78]. Similar favorable results have been reported in other smaller series[79,80]. The key advantage of the BASILICA procedure is the possibility of avoiding stent implantation, which mitigates the need for prolonged DAPT therapy and avoids potential stent-related complications such as under-expansion or restenosis.
Management of coronary obstruction
The diagnosis of CO is usually immediately apparent and can be confirmed by aortography and ECG evaluation, with echocardiography utilized to evaluate for regional wall motion abnormalities if doubt persists. CO usually occurs following post-dilatation or during initial deployment with a BEV. If CO occurs following deployment of a SEV, then, depending on the type of valve and stage of deployment, the device can be recaptured immediately to restore flow. If repositioning is not feasible, then immediate stenting should be attempted and if unsuccessful, then an inflated balloon or snare can be used to pull up a SEV into the ascending aorta. Hemodynamic support, including use of ECMO, should be considered early to facilitate an interventional solution to the CO. If, however, the situation cannot be resolved percutaneously, then emergent open-heart bypass remains the mainstay of treatment[50].
PARAVALVULAR LEAK
Post-TAVR paravalvular leak (PVL) arises due to incomplete device apposition against the native aortic annulus. Residual moderate-severe PVL is a strong independent predictor for mortality[1-3]. Even mild PVL, which was observed in 29.4% and 33.9% of subjects at one year post-TAVR in the PARTNER3[6] and Evolut[7] low-risk trials, respectively, was associated with elevated mortality at two and five years post TAVR[81,82]. Therefore, particularly for younger patients undergoing TAVR, the aim should be to achieve minimal to no residual leak.
Minimizing risk of paravalvular leak
Accurate pre-procedural CT analysis should prevent valve under-expansion and can also be used to identify high-risk features such as heavy annular and/or leaflet calcification, which may lead to valve under-expansion or malpositioning [Figure 1][83]. In subjects where heavy calcification is expected to increase the risk of significant paravalvular leak, newer generation valves should be considered. The Sapien 3 ultra (Edwards LifeSciences), Evolut Plus (Medtronic), and ACURATE neo 2 (Boston Scientific) valves all have an additional sealing skirt and the NAVITOR (Abbott) valve has a dynamic sealing skirt added to minimize PVL. Therefore, detailed pre-procedural CT evaluation combined with appropriate device selection plays a key role in minimizing PVL[84].
Management of paravalvular leak
When faced with post-implant aortic regurgitation in the catheterization laboratory, it is important to differentiate between PVL and guide wire-induced central aortic regurgitation. Retraction of the guide wire or exchanging the stiff wire for a softer pigtail catheter may clarify the diagnosis. Currently, the gold-standard for evaluation of PVL remains echocardiography[85], however transthoracic visualization may be sub-optimal and the use of intra-procedural transesophageal echocardiography is diminishing as minimalist TAVR procedures with conscious sedation are being widely adopted[20,86].
Conventional aortography, although convenient, is only helpful at the extremes of AR severity[85]. Quantitative video densitometry may provide a better evaluation of AR severity, but as with simple aortography it is limited in its ability to distinguish between central or paravalvular regurgitation. Several hemodynamic indices have been proposed[87] [Table 1], with the AR index (calculated as LVEDP-DPB/SBP) being the most widely adopted[88]. However, hemodynamic indices may be limited by heart-rate variations, atrial fibrillation, and altered hemodynamic conditions due to sedation and/or anesthesia. Therefore, in cases of doubt, intra-procedural echocardiography with color and doppler evaluation is still recommended[85].
Overview of hemodynamic indices to quantify extent of aortic regurgitation during transcatheter aortic valve replacement
| Index | Formula | Cut-off |
| Aortic regurgitation index (ARI) | ARI = (DD/systolic aortic pressure) × 100 | ARI < 25 associated with increased mortality |
| Aortic regurgitation index ratio | ARI ratio = ARI post-implant/ARI pre-implant | ARI ratio < 0.60 associated with increased mortality |
| Diastolic delta (DD) | Diastolic aortic pressure - left ventricular end diastolic pressure | DD < 18 mmHg associated with increased mortality |
| Heart-rate (HR) adjusted diastolic delta | (DD/heart rate) × 80 | HR-DD < 25 associated with increased mortality |
Treatment of PVL
Balloon post-dilatation represents the main stay of treatment with balloon sizing based on pre-procedural CT measurements of the annulus. The use of repeated aggressive balloon dilatation to correct PVL must be balanced against the risk of subsequent aortic injury, annular rupture, or stroke, especially in patients with heavy or nodular calcification. When faced with this scenario, the degree of pre-TAVR AR can be a useful guide as to how aggressively post-dilatation should be performed. Patients with mixed aortic valve disease have more compliant ventricles and can tolerate post-procedural AR better than those with smaller, stiffer ventricles[89].
If despite balloon dilatation, PVL is severe or is associated with significant hemodynamic compromise, then a second THV can be implanted as a TAVR-in-TAVR procedure, with a BEV preferred[48]. In more stable cases, or if discovered later post-procedurally, then elective closure with vascular plugs should be considered[43].
ACUTE KIDNEY INJURY
Post-TAVR acute kidney injury (AKI) can be diagnosed by measuring the increase in serum creatinine or reduction in urine output as specified in the VARC-2 criteria, which are based on the Acute Kidney Injury Network definitions[1]. Depending on the cohort, the overall incidence of AKI ranges 10%-40%, with stage 3 AKI observed in around 1% of patients undergoing TAVR[3,90-92]. Encouragingly, the incidence of AKI in contemporary cohorts is down to around 10%, which may reflect the changes in clinical profile of patients undergoing TAVR or be related to improvements in procedural techniques[92]. However, numerous studies have consistently shown that the development of post-TAVR AKI is associated with adverse acute and longer-term morbidity, mortality, and quality of life[3,90-92]. A recent report of 107,814 patients from the society of thoracic surgeons TAVR registry in the USA demonstrated an elevated risk of one-year mortality associated with stage 1 (HR = 2.7, 95%CI: 2.5-2.8), stage 2 (HR = 10.4, 95%CI: 7.0-15.4), and stage 3 (HR = 7.0, 95%CI: 6.0-8.2) AKI[92].
Multiple patient-related and procedural factors are implicated in the development of post-TAVR AKI. Baseline renal dysfunction is one of the strongest independent risk factors for the long-term mortality and development of post-TAVR AKI[90,92,93]. In addition, patients with cardiovascular and non-cardiovascular co-morbidities, including anemia, diabetes, chronic obstructive pulmonary disease, severe inflammatory response syndrome, and aortic or peripheral vascular disease, are at increased risk[90,93-96].
During the TAVR procedure, the kidneys can be prone to injury either due to hemodynamic instability during rapid pacing, significant bleeding, and prosthesis deployment or due to embolism of atherosclerotic or calcific micro-fragments during catheter manipulation. Managing complications may require an increased use of contrast, conversion to general anesthesia, and open-heart surgery or requirement for red blood cell transfusions, which can further increase the risk of AKI[93]. Furthermore, choice of TAVR valve and access route can be important with a higher incidence of AKI observed following the use of self-expandable vales and direct apical or aortic access[95].
The association between the type and volume of contrast agent used and subsequent risk of AKI following TAVR is less clear. Use of a low-osmolar vs. iso-osmolar contrast agent during TAVR did not alter the incidence of AKI. Similarly, the total contrast volume used during the procedure was found to have no impact on development of AKI in several studies. However, when contrast volume was indexed to baseline renal function, then the amount of contrast used was predictive of both AKI and mortality[97,98].
Prevention of AKI
Accurate identification of patients at high risk of developing AKI should prompt the use of appropriate prevention strategies. Ratios of contrast volume to glomerular filtration rate of > 3.2 and threshold value of contrast volume × serum creatinine to body weight of 2.7 were cutoffs identified by predicting mortality and development of AKI[97,98].
For these high-risk patients, adequate pre-hydration, particularly when combined with close monitoring of volume status either conventionally or with the use of modern techniques such as RenalGuard system (RenalGuard Solutions Inc, Milford, MA), is an important first step[99,100].
For patients with baseline renal dysfunction, contrast sparing strategies including the use of alternative imaging modalities, such as magnetic resonance imaging (MRI) or 3D transesophageal echocardiography, for pre-procedural planning and intra-procedural guidance should be considered. Low-contrast volume CT protocols which provide adequate assessment of aortic and peripheral vessels have been described[101-103]. Pre-procedural TAVR CT can be performed with cardiac gating to evaluate coronary arteries, obviating the need for invasive angiography and thereby further reducing the total contrast volume administered pre-procedurally. Alternatively, echocardiography, gadolinium-free cardiac magnetic resonance tomography, and fusion angiography can be used with procedural adaptions to perform an almost zero-contrast procedure[104,105]. If small volumes of contrast are required, then the use of a non-ionic iso-osmolar contrast media is recommended.
Meticulous attention should be given to vascular access to avoid vascular complications, especially major bleeding. When necessary, a restrictive transfusion policy should be adopted. The use of embolic protection devices may limit the burden of embolic damage to the kidneys, although future studies in this field are awaited.
STROKE
Despite improvements in device technologies, operator experience, and a lowering in the risk profile of patients, the rate of clinically relevant post-TAVR strokes remains static around 2% and seriously impacts upon quality of life, morbidity, and mortality[106,107]. Additionally, silent cerebral ischemic lesions detected by diffusion weighted MRI have been identified in up to 80% of patients undergoing TAVR[108-110]. Although the mid- and long-term consequence of these silent infarctions is debated, some studies have suggested an association with longer-term neurocognitive changes and dementia.
Acute cerebrovascular events: < 24 h
The majority of post-TAVR strokes occur within the first 24 h and are due to embolization of debris composed of thrombus, calcification, atheromatous plaques, vascular endothelium, or tissue valve fragments[111]. Consequently, cerebral embolic protection devices (CEPD) were designed to capture and/or deflect this debris and thereby reduce the incidence of peri-procedural stroke. Currently, only two devices with Conformite Europeenne mark are commercially available, the Sentinel™ Cerebral Protection System (Boston Scientific, Natick, MA) and TriGUARD™ (Keystone Heart Ltd, Herzliya, Israel) devices. The Sentinel CPS is a 6 Fr system, which only covers the brachiocephalic and left common carotid arteries. In contrast, the TriGUARD device is a larger 9 Fr system, which provides more complete coverage of all the supra-aortic vessels. Globally, the use of CEPDs has gradually increased with data from the Society of Thoracic Surgeons-Transcatheter Valvular Therapies registry revealing that a CEPD was used during 13% of TAVR procedures by 2019[112,113]. Both devices require the use of an alternative arterial access and may potentially interfere with valve advancement and manipulation inside the aortic arch. Newer generation devices such as the Emblok device, which incorporates a radiopaque 4 Fr guiding pigtail catheter in the device system, mitigates the need for an additional access[114].
Generally, the use of CEPDs during TAVR has not been shown to reduce the incidence of in-hospital stroke[112,113,115,116]. In contrast, data regarding the impact of CEPDs on MRI-defined lesion volume are conflicting[117-119]; however, a recent updated meta-analysis did not find any significant difference associated with CEPD use on MRI lesion volume or number of new ischemic lesions[115]. Further definitive answers regarding the effectiveness of CEPDs for stroke reduction are expected with the results of the PROTECTED TAVR (Stroke Protection With Sentinel During Transcatheter Aortic Valve Replacement) trial (NCT: 04149535) enrolling 3000 patients randomized 1:1 to TAVR with or without the Sentinel protection device.
Even with a CEPD deployed, a significant burden of silent cerebral events can still occur, which highlights the importance of caution during wire, catheter, and delivery system exchanges and manipulations. Heparin administration to reduce peri-procedural thrombosis should be activated clotting time guided, aiming between 250 and 300 s. In high-bleeding-risk patients who develop intra-procedural or early post-operative acute ischemic stroke, where a filling defect of a large intra-cranial vessel is identified, mechanical thrombectomy can be performed[120,121].
Late cerebrovascular events: 24 h to 30 days
Late cerebrovascular events (CVE) mainly arise from cardio-embolic sources, with atrial fibrillation (AF) being the commonest cause[122]. Patients with AF undergoing TAVR usually have multiple co-morbidities with associated elevated thrombotic and bleeding risks, which requires specific attention[123]. Heparin reversal with protamine should be used cautiously and alternative methods to achieve hemostasis are preferred. In certain cases, simultaneous implantation of a left atrial appendage occluder device during TAVR has been performed and data from the WATCH-TAVR (NCT: 03173534) trial, which randomizes patients to TAVR + medical therapy or TAVR + WATCHMAN device, are expected to provide further insights. Current ESC/EACTS guidelines and other expert consensus recommend post-procedural oral anticoagulation therapy alone or in combination with single anti-platelet agent depending on bleeding risk profile[124,125].
Finally, leaflet thrombosis or sub-clinical leaflet thrombosis is increasingly being recognized as a potential source of late CVE, although reports are conflicting[126]. Although routine post-TAVR anticoagulation reduced the incidence of sub-clinical and leaflet thrombosis, this did not translate into a net clinical benefit due to the increased bleeding risk[127]. Taken together, these findings suggest that post-procedural anticoagulation therapy should be limited to those patients with a pre-existing indication.
PACEMAKER IMPLANTATION
The incidence of permanent pacemaker implantation (PPI) remains significant in contemporary cohorts[128]. As TAVR expands towards lower-risk younger patients, a shorter hospital stay and earlier discharge becomes more favorable. Given that the risk of developing high-grade atrio-ventricular block requiring pacing is often unpredictable, individualized risk assessment is necessary to identify high-risk electrical, anatomical, and procedural features[129]. Patients with pre-existing conduction disease such as right bundle branch block and prolonged PR-interval confer an elevated risk of requiring both early and late PPI[130,131]. Increased calcium burden and distribution particularly in the DLZ and LVOT, as well as a deeper implantation, are important procedural considerations, which are associated with elevated post-procedural PPI rates[132,133].
During the procedure, rapid atrial pacing can be performed to risk stratify patients. Those who develop atrial-pacing-induced Wenckebach atrioventricular (AV) block are at high risk for PPI, especially when AV block occurs at lower rates of atrial pacing. Among 284 patients who were evaluated, those who did not develop Wenckebach AV block had a negative predictive value of 98.7% for PPI[134].
Choice of valve prosthesis can have an impact, with lower rates of PPI seen with the use of BEV compared to SEV[128]. Latest generation valves such as the ACURATE neo, with its low radial strength, were associated with a low PPI rate of 8.3% in a 1000 patients[135]. Furthermore, procedural techniques have been developed which aim to precisely and systematically achieve a higher implantation with the aim of further reducing permanent pacemaker implantation rates. The cusp overlap technique involves overlapping the left and right coronary cusps to isolate the non-coronary cusp[136]. This is achieved by rotating the C-arm in a right anterior oblique caudal direction and the optimal projection can be determined from pre-procedural CT. The key advantage of this projection is that both the delivery catheter and aortic cusps can be aligned, delivery catheter parallax is eliminated, and the LVOT is not foreshortened, which allows for a more precise and higher implantation depth. Early registry data are promising with dramatically lower permanent pacemaker (PPM) implantation rates (4%-7%) observed when using the cusp overlap technique with different THV[137,138]. Further lowering of PPM rates, particularly with self-expandable devices, can be achieved using the minimizing depth according to the membranous septum technique[139]. This patient-specific approach utilizes the pre-procedural CT to measure the membranous septum, aiming to achieve an implantation depth less than the length of the membranous septum. When this technique was applied to a consecutive series of patients prospectively, pacemaker implantation rates fell from 9.7% to 3% with rates of left bundle branch block falling from 25.8% to 9%[139].
At the end of the procedure, consideration must be given to the duration and type of monitoring required[129]. An algorithmic approach to select which patients require prolonged hospital or ambulatory ECG monitoring may minimize the potentially fatal complication of post-discharge high-grade atrio-ventricular block[129,140].
CONCLUSION
The evolution of device technology and operator experience has dramatically changed the safety profile of TAVR. However, the need to prevent and appropriately manage peri-procedural complications remains ever important, especially as TAVR expands towards lower surgical risk populations. Meticulous pre-procedural planning is critical to ensuring the risk for complications is minimized.
DECLARATIONS
Authors’ contributionsConception and design of review article: Khokhar AA, Giannini F, Dudek D, Colombo A
Writing of content and creation of figures: Khokhar AA, Ruggiero R, Chandra K, D’Agostino A, Toselli M, Mangieri A, Colombo A
Critical review of article: Khokhar AA, Toselli M, Mangieri A, Dudek D, Colombo A, Giannini F
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestMangieri A & Dudek D received scientific grant from Boston Scientific Inc. Other authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012;60:1438-54.
2. Généreux P, Webb JG, Svensson LG, et al. PARTNER Trial Investigators. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J Am Coll Cardiol 2012;60:1043-52.
3. Arnold SV, Zhang Y, Baron SJ, et al. Impact of short-term complications on mortality and quality of life after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2019;12:362-9.
4. Ueshima D, Fovino LN, D'Amico G, Brener SJ, Esposito G, Tarantini G. Transcatheter versus surgical aortic valve replacement in low- and intermediate-risk patients: an updated systematic review and meta-analysis. Cardiovasc Interv Ther 2019;34:216-25.
5. Rahhab Z, Ramdat Misier K, El Faquir N, et al. Vascular complications after transfemoral transcatheter aortic valve implantation: a systematic review and meta-analysis. Structural Heart 2020;4:62-71.
6. Mack MJ, Leon MB, Thourani VH, et al. PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695-705.
7. Popma JJ, Deeb GM, Yakubov SJ, et al. Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706-15.
8. Blanke P, Weir-McCall JR, Achenbach S, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): an expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2019;13:1-20.
9. Mangieri A, Laricchia A, Montalto C, et al. Patient selection, procedural planning and interventional guidance for transcatheter aortic valve intervention. Minerva Cardiol Angiol 2021;69:671-83.
10. Francone M, Budde RPJ, Bremerich J, et al. CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR). Eur Radiol 2020;30:2627-50.
11. Junquera L, Urena M, Latib A, et al. Comparison of transfemoral versus transradial secondary access in transcatheter aortic valve replacement. Circ Cardiovasc Interv 2020;13:e008609.
12. Allende R, Urena M, Cordoba JG, et al. Impact of the use of transradial versus transfemoral approach as secondary access in transcatheter aortic valve implantation procedures. Am J Cardiol 2014;114:1729-34.
13. Di Mario C, Goodwin M, Ristalli F, et al. A prospective registry of intravascular lithotripsy-enabled vascular access for transfemoral transcatheter aortic valve replacement. JACC Cardiovasc Interv 2019;12:502-4.
14. Faroux L, Junquera L, Mohammadi S, et al. Femoral versus nonfemoral subclavian/carotid arterial access route for transcatheter aortic valve replacement: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e017460.
15. Beurtheret S, Karam N, Resseguier N, et al. Femoral versus nonfemoral peripheral access for transcatheter aortic valve replacement. J Am Coll Cardiol 2019;74:2728-39.
16. Dahle TG, Kaneko T, McCabe JM. Outcomes following subclavian and axillary artery access for transcatheter aortic valve replacement: Society of the Thoracic Surgeons/American College of Cardiology TVT Registry Report. JACC Cardiovasc Interv 2019;12:662-9.
17. Greenbaum AB, Babaliaros VC, Chen MY, et al. Transcaval access and closure for transcatheter aortic valve replacement: a prospective investigation. J Am Coll Cardiol 2017;69:511-21.
18. Lederman RJ, Babaliaros VC, Rogers T, et al. The fate of transcaval access tracts: 12-month results of the prospective NHLBI transcaval transcatheter aortic valve replacement study. JACC Cardiovasc Interv 2019;12:448-56.
19. Elbaz-Greener G, Zivkovic N, Arbel Y, Radhakrishnan S, Fremes SE, Wijeysundera HC. Use of two-dimensional ultrasonographically guided access to reduce access-related complications for transcatheter aortic valve replacement. Can J Cardiol 2017;33:918-24.
20. Mangieri A, Khokhar A, Giannini F, Colombo A. Transcatheter aortic valve replacement from a single vascular access: an ultra-minimalist approach. Clin Res Cardiol 2021;110:469-71.
21. Barbash IM, Barbanti M, Webb J, et al. Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation. Eur Heart J 2015;36:3370-9.
22. Dimitriadis Z, Scholtz W, Börgermann J, et al. Impact of closure devices on vascular complication and mortality rates in TAVI procedures. Int J Cardiol 2017;241:133-7.
23. Ott I, Shivaraju A, Schäffer NR, et al. Parallel suture technique with ProGlide: a novel method for management of vascular access during transcatheter aortic valve implantation (TAVI). EuroIntervention 2017;13:928-34.
24. Tarantini G. MANTA dedicated large-bore vessel closure device. Circ Cardiovasc Interv 2019;12:e008203.
25. Barbanti M, Capranzano P, Ohno Y, et al. Comparison of suture-based vascular closure devices in transfemoral transcatheter aortic valve implantation. EuroIntervention 2015;11:690-7.
26. Berti S, Bedogni F, Giordano A, et al. Italian Society of Interventional Cardiology-GISE†. Efficacy and safety of ProGlide versus prostar XL vascular closure devices in transcatheter aortic valve replacement: the RISPEVA registry. J Am Heart Assoc 2020;9:e018042.
27. Van Mieghem NM, Latib A, van der Heyden J, et al. Percutaneous plug-based arteriotomy closure device for large-bore access: a multicenter prospective study. JACC Cardiovasc Interv 2017;10:613-9.
28. Wood DA, Krajcer Z, Sathananthan J, et al. SAFE MANTA Study Investigators. Pivotal clinical study to evaluate the safety and effectiveness of the MANTA percutaneous vascular closure device. Circ Cardiovasc Interv 2019;12:e007258.
29. Nuis RJ, Wood D, Kroon H, et al. Frequency, impact and predictors of access complications with plug-based large-bore arteriotomy closure - a patient level meta-analysis. Cardiovasc Revasc Med 2021; doi: 10.1016/j.carrev.2021.02.017.
30. Kroon HG, Tonino PAL, Savontaus M, et al. Dedicated plug based closure for large bore access - the MARVEL prospective registry. Catheter Cardiovasc Interv 2021;97:1270-8.
31. van Wiechen MP, Tchétché D, Ooms JF, et al. Suture- or plug-based large-bore arteriotomy closure: a pilot randomized controlled trial. JACC Cardiovasc Interv 2021;14:149-57.
32. Pasic M, Unbehaun A, Buz S, Drews T, Hetzer R. Annular rupture during transcatheter aortic valve replacement: classification, pathophysiology, diagnostics, treatment approaches, and prevention. JACC Cardiovasc Interv 2015;8:1-9.
33. Schymik G, Heimeshoff M, Bramlage P, et al. Ruptures of the device landing zone in patients undergoing transcatheter aortic valve implantation: an analysis of TAVI Karlsruhe (TAVIK) patients. Clin Res Cardiol 2014;103:912-20.
34. Aminian A, Lalmand J, Dolatabadi D. Late contained aortic root rupture and ventricular septal defect after transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2013;81:E72-5.
35. Barbanti M, Yang TH, Rodès Cabau J, et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013;128:244-53.
36. Hansson NC, Nørgaard BL, Barbanti M, et al. The impact of calcium volume and distribution in aortic root injury related to balloon-expandable transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr 2015;9:382-92.
37. Barbanti M. Avoiding coronary occlusion and root rupture in TAVI - the role of pre-procedural imaging and prosthesis selection. Interv Cardiol 2015;10:94-7.
38. Tsuda M, Mizote I, Mukai T, Sakata Y. Aortic root rupture during balloon-expandable transcatheter aortic valve replacement in a patient without recognized risk factors for aortic root rupture: a case report. Eur Heart J Case Rep 2020;4:1-4.
39. Pignatelli A, Pestrichella V, Contegiacomo G, Navarese EP. Percutaneous treatment of aortic root rupture after transcatheter aortic valve replacement procedure. J Cardiovasc Med (Hagerstown) 2020;21:158-60.
40. Nagaraja V, Ratib K, Nolan J. Annular rupture successfully salvaged by valve-in-valve implantation. Structural Heart 2019;3:160-2.
41. Azarrafiy R, Albuquerque FN, Carrillo RG, Cohen MG. Coil embolization to successfully treat annular rupture during transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2018;92:1205-8.
42. Alkhouli M, Carpenter E, Tarabishy A, Sengupta P. Annular rupture during transcatheter aortic valve replacement: novel treatment with amplatzer vascular plugs. Eur Heart J 2018;39:714-5.
43. Conte SM, Kearney K, Jain P, et al. Plugging paravalvular leak in transcatheter aortic valves. JACC Case Rep 2019;1:696-702.
44. Takei M, Hasegawa T, Ohtsubo S, Takahashi T. Effusion in transverse sinus: a primary echocardiographic sign for aortic annular rupture in transcatheter aortic valve replacement. J Echocardiogr 2021;19:121-2.
45. Langer NB, Hamid NB, Nazif TM, et al. Injuries to the aorta, aortic annulus, and left ventricle during transcatheter aortic valve replacement: management and outcomes. Circ Cardiovasc Interv 2017;10:e004735.
46. Selhane D, Urena-alcazar M, Veugeois A, et al. Peri-procedural tamponade following TAVI: Incidence, predictors and impact on outcome. Arch Cardiovasc Dis Suppl 2019;11:70.
47. Hensey M, Daniels D, Wood D, Webb JG. Early experience with a purpose-designed temporary pacing guidewire for transcatheter valve implantation. EuroIntervention 2019;15:e508-9.
48. Makkar RR, Jilaihawi H, Chakravarty T, et al. Determinants and outcomes of acute transcatheter valve-in-valve therapy or embolization: a study of multiple valve implants in the U.S. PARTNER trial (Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve). J Am Coll Cardiol 2013;62:418-30.
49. Kim WK, Schäfer U, Tchetche D, et al. Incidence and outcome of peri-procedural transcatheter heart valve embolization and migration: the TRAVEL registry (TranscatheteR HeArt Valve EmboLization and Migration). Eur Heart J 2019;40:3156-65.
50. Eggebrecht H, Vaquerizo B, Moris C, et al. European Registry on Emergent Cardiac Surgery during TAVI (EuRECS-TAVI). Incidence and outcomes of emergent cardiac surgery during transfemoral transcatheter aortic valve implantation (TAVI): insights from the European Registry on Emergent Cardiac Surgery during TAVI (EuRECS-TAVI). Eur Heart J 2018;39:676-84.
51. Ibebuogu UN, Giri S, Bolorunduro O, et al. Review of reported causes of device embolization following trans-catheter aortic valve implantation. Am J Cardiol 2015;115:1767-72.
52. Nkomo VT, Suri RM, Pislaru SV, et al. Delayed transcatheter heart valve migration and failure. JACC Cardiovasc Imaging 2014;7:960-2.
53. Radu C, Raffoul R, Brochet E, Himbert D. Delayed migration of a transfemorally implanted aortic bioprosthesis. J Thorac Cardiovasc Surg 2012;143:e1-3.
54. Vendrik J, van den Boogert TPW, Koch KT, Baan J Jr. Balloon-expandable TAVR prosthesis dislocates into the ascending aorta. JACC Case Rep 2019;1:101-4.
55. Fournier S, Monney P, Roguelov C, et al. How should I treat an Edwards SAPIEN 3 aortic valve embolisation during a transaortic transcatheter aortic valve implantation? EuroIntervention 2017;13:495-8.
56. Ussia GP, Barbanti M, Ramondo A, et al. The valve-in-valve technique for treatment of aortic bioprosthesis malposition an analysis of incidence and 1-year clinical outcomes from the italian CoreValve registry. J Am Coll Cardiol 2011;57:1062-8.
57. Alkhouli M, Sievert H, Rihal CS. Device embolization in structural heart interventions: incidence, outcomes, and retrieval techniques. JACC Cardiovasc Interv 2019;12:113-26.
58. Tay EL, Gurvitch R, Wijeysinghe N, et al. Outcome of patients after transcatheter aortic valve embolization. JACC Cardiovasc Interv 2011;4:228-34.
59. Ribeiro HB, Nombela-Franco L, Urena M, et al. Coronary obstruction following transcatheter aortic valve implantation: a systematic review. JACC Cardiovasc Interv 2013;6:452-61.
60. Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013;62:1552-62.
61. Dvir D, Leipsic J, Blanke P, et al. Coronary obstruction in transcatheter aortic valve-in-valve implantation: preprocedural evaluation, device selection, protection, and treatment. Circ Cardiovasc Interv 2015;8:e002079.
62. Jabbour RJ, Tanaka A, Finkelstein A, et al. Delayed coronary obstruction after transcatheter aortic valve replacement. J Am Coll Cardiol 2018;71:1513-24.
63. Ribeiro HB, Rodés-Cabau J, Blanke P, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J 2018;39:687-95.
64. De Marco F, Casenghi M, Spagnolo P, et al. A patient-specific algorithm to achieve commissural alignment with Acurate Neo: the sextant technique. Catheter Cardiovasc Interv 2021;98:E847-54.
65. Tang GHL, Zaid S, Fuchs A, et al. Alignment of transcatheter aortic-valve neo-commissures (ALIGN TAVR): impact on final valve orientation and coronary artery overlap. JACC Cardiovasc Interv 2020;13:1030-42.
66. Kitamura M, Wilde J, Gohmann R, et al. Commissural alignment of the ACURATE neo valve in transcatheter aortic valve replacement. JACC Cardiovasc Interv 2021;14:1740-2.
67. Wong I, Bieliauskas G, De Backer O, Søndergaard L. Technical considerations for transcatheter aortic valve replacement with ACURATE neo2. JACC Cardiovasc Interv 2021;14:224-6.
68. Mercanti F, Rosseel L, Neylon A, et al. Chimney stenting for coronary occlusion during TAVR: insights from the chimney registry. JACC Cardiovasc Interv 2020;13:751-61.
69. Palmerini T, Chakravarty T, Saia F, et al. Coronary protection to prevent coronary obstruction during TAVR: a multicenter international registry. JACC Cardiovasc Interv 2020;13:739-47.
70. Abramowitz Y, Chakravarty T, Jilaihawi H, et al. Clinical impact of coronary protection during transcatheter aortic valve implantation: first reported series of patients. EuroIntervention 2015;11:572-81.
71. Pighi M, Lunardi M, Pesarini G, et al. Intravascular ultrasound assessment of coronary ostia following valve-in-valve transcatheter aortic valve implantation. EuroIntervention 2021;16:1148-51.
72. Khan JM, Greenbaum AB, Babaliaros VC, et al. The BASILICA trial: prospective multicenter investigation of intentional leaflet laceration to prevent TAVR coronary obstruction. JACC Cardiovasc Interv 2019;12:1240-52.
73. Lederman RJ, Babaliaros VC, Rogers T, et al. Preventing coronary obstruction during transcatheter aortic valve replacement: from computed tomography to BASILICA. JACC Cardiovasc Interv 2019;12:1197-216.
74. Khan JM, Dvir D, Greenbaum AB, et al. Transcatheter laceration of aortic leaflets to prevent coronary obstruction during transcatheter aortic valve replacement: concept to first-in-human. JACC Cardiovasc Interv 2018;11:677-89.
75. Komatsu I, Mackensen GB, Aldea GS, Reisman M, Dvir D. Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. Part 2: how to perform BASILICA. EuroIntervention 2019;15:55-66.
76. Komatsu I, Tang GHL, Leipsic J, et al. Distribution of C-arm projections in native and bioprosthetic aortic valves cusps: implication for BASILICA procedures. Catheter Cardiovasc Interv 2021;97:E580-7.
77. Tang GHL, Komatsu I, Tzemach L, et al. Risk of coronary obstruction and the need to perform BASILICA: the VIVID classification. EuroIntervention 2020;16:e757-9.
78. Khan JM, Babaliaros VC, Greenbaum AB, et al. Preventing coronary obstruction during transcatheter aortic valve replacement: results from the multicenter international BASILICA registry. JACC Cardiovasc Interv 2021;14:941-8.
79. Westermann D, Ludwig S, Kalbacher D, et al. Prevention of coronary obstruction in patients at risk undergoing transcatheter aortic valve implantation: the Hamburg BASILICA experience. Clin Res Cardiol 2021;110:1900-11.
80. Kitamura M, Majunke N, Holzhey D, et al. Systematic use of intentional leaflet laceration to prevent TAVI-induced coronary obstruction: feasibility and early clinical outcomes of the BASILICA technique. EuroIntervention 2020;16:682-90.
81. Makkar RR, Thourani VH, Mack MJ, et al. PARTNER 2 Investigators. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med 2020;382:799-809.
82. Van Belle E, Vincent F, Labreuche J, et al. Balloon-expandable versus self-expanding transcatheter aortic valve replacement: a propensity-matched comparison from the FRANCE-TAVI registry. Circulation 2020;141:243-59.
83. Généreux P, Head SJ, Hahn R, et al. Paravalvular leak after transcatheter aortic valve replacement: the new Achilles’ heel? J Am Coll Cardiol 2013;61:1125-36.
84. Modolo R, Chang CC, Abdelghani M, et al. Quantitative assessment of acute regurgitation following TAVR: a multicenter pooled analysis of 2,258 valves. JACC Cardiovasc Interv 2020;13:1303-11.
85. Zoghbi WA, Asch FM, Bruce C, et al. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2019;32:431-75.
86. Akodad M, Lefèvre T. TAVI: simplification is the ultimate sophistication. Front Cardiovasc Med 2018;5:96.
87. Abdelghani M, Soliman OI, Schultz C, Vahanian A, Serruys PW. Adjudicating paravalvular leaks of transcatheter aortic valves: a critical appraisal. Eur Heart J 2016;37:2627-44.
88. Sinning JM, Hammerstingl C, Vasa-Nicotera M, et al. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol 2012;59:1134-41.
89. Chahine J, Kadri AN, Gajulapalli RD, et al. Outcomes of transcatheter aortic valve replacement in mixed aortic valve disease. JACC Cardiovasc Interv 2019;12:2299-306.
90. Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010;31:865-74.
91. Takagi H, Niwa M, Mizuno Y, Goto SN, Umemoto T. All-Literature Investigation of Cardiovascular Evidence Group. Incidence, predictors, and prognosis of acute kidney injury after transcatheter aortic valve implantation: a summary of contemporary studies using Valve Academic Research Consortium definitions. Int J Cardiol 2013;168:1631-5.
92. Julien HM, Stebbins A, Vemulapalli S, et al. Incidence, predictors, and outcomes of acute kidney injury in patients undergoing transcatheter aortic valve replacement: insights from the Society of Thoracic Surgeons/American College of Cardiology National Cardiovascular Data Registry-Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv 2021;14:e010032.
93. Nuis RJ, Van Mieghem NM, Tzikas A, et al. Frequency, determinants, and prognostic effects of acute kidney injury and red blood cell transfusion in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2011;77:881-9.
94. Elhmidi Y, Bleiziffer S, Piazza N, et al. Incidence and predictors of acute kidney injury in patients undergoing transcatheter aortic valve implantation. Am Heart J 2011;161:735-9.
95. Kong WY, Yong G, Irish A. Incidence, risk factors and prognosis of acute kidney injury after transcatheter aortic valve implantation. Nephrology (Carlton) 2012;17:445-51.
96. Chandrasekhar J, Sartori S, Mehran R, et al. Incidence, predictors, and outcomes associated with acute kidney injury in patients undergoing transcatheter aortic valve replacement: from the BRAVO-3 randomized trial. Clin Res Cardiol 2021;110:649-57.
97. Yamamoto M, Hayashida K, Mouillet G, et al. Renal function-based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovasc Interv 2013;6:479-86.
98. Giannini F, Latib A, Jabbour RJ, et al. The ratio of contrast volume to glomerular filtration rate predicts acute kidney injury and mortality after transcatheter aortic valve implantation. Cardiovasc Revasc Med 2017;18:349-55.
99. Barbanti M, Gulino S, Capranzano P, et al. Acute kidney injury with the RenalGuard system in patients undergoing transcatheter aortic valve replacement: the PROTECT-TAVI trial (PROphylactic effecT of furosEmide-induCed diuresis with matched isotonic intravenous hydraTion in Transcatheter Aortic Valve Implantation). JACC Cardiovasc Interv 2015;8:1595-604.
100. Wang Y, Guo Y. RenalGuard system and conventional hydration for preventing contrast-associated acute kidney injury in patients undergoing cardiac interventional procedures: a systematic review and meta-analysis. Int J Cardiol 2021;333:83-9.
101. Suchá D, Kino A, Bogart K, et al. Effect of low contrast medium-dose CTA on device sizing and access vessel assessment for TAVR. Eur J Radiol 2020;124:108826.
102. Pulerwitz TC, Khalique OK, Nazif TN, et al. Very low intravenous contrast volume protocol for computed tomography angiography providing comprehensive cardiac and vascular assessment prior to transcatheter aortic valve replacement in patients with chronic kidney disease. J Cardiovasc Comput Tomogr 2016;10:316-21.
103. Cavallo AU, Patterson AJ, Thomas R, et al. Low dose contrast CT for transcatheter aortic valve replacement assessment: results from the prospective SPECTACULAR study (spectral CT assessment prior to TAVR). J Cardiovasc Comput Tomogr 2020;14:68-74.
104. Higuchi R, Tobaru T, Hagiya K, et al. Renoprotective transcatheter aortic valve implantation without contrast media. Int Heart J 2018;59:1469-72.
105. Castriota F, Nerla R, Micari A, Squeri A, Cremonesi A. Contrast-zero transcatheter aortic valve replacement for patients with severe renal dysfunction: a single-center experience. JACC Cardiovasc Interv 2018;11:820-2.
106. Huded CP, Tuzcu EM, Krishnaswamy A, et al. Association between transcatheter aortic valve replacement and early postprocedural stroke. JAMA 2019;321:2306-15.
107. Kapadia SR, Huded CP, Kodali SK, et al. PARTNER Trial Investigators. Stroke after surgical versus transfemoral transcatheter aortic valve replacement in the PARTNER trial. J Am Coll Cardiol 2018;72:2415-26.
108. Pagnesi M, Martino EA, Chiarito M, et al. Silent cerebral injury after transcatheter aortic valve implantation and the preventive role of embolic protection devices: a systematic review and meta-analysis. Int J Cardiol 2016;221:97-106.
109. Spaziano M, Francese DP, Leon MB, Généreux P. Imaging and functional testing to assess clinical and subclinical neurological events after transcatheter or surgical aortic valve replacement: a comprehensive review. J Am Coll Cardiol 2014;64:1950-63.
110. Kahlert P, Al-Rashid F, Döttger P, et al. Cerebral embolization during transcatheter aortic valve implantation: a transcranial Doppler study. Circulation 2012;126:1245-55.
111. Van Mieghem NM, Schipper ME, Ladich E, et al. Histopathology of embolic debris captured during transcatheter aortic valve replacement. Circulation 2013;127:2194-201.
112. Stachon P, Kaier K, Heidt T, et al. The use and outcomes of cerebral protection devices for patients undergoing transfemoral transcatheter aortic valve replacement in clinical practice. JACC Cardiovasc Interv 2021;14:161-8.
113. Butala NM, Makkar R, Secemsky EA, et al. Cerebral embolic protection and outcomes of transcatheter aortic valve replacement: results from the transcatheter valve therapy registry. Circulation 2021;143:2229-40.
114. Latib A, Mangieri A, Vezzulli P, et al. First-in-man study evaluating the Emblok embolic protection system during transcatheter aortic valve replacement. JACC Cardiovasc Interv 2020;13:860-8.
115. Ahmad Y, Howard JP. Meta-analysis of usefulness of cerebral embolic protection during transcatheter aortic valve implantation. Am J Cardiol 2021;146:69-73.
116. Kapadia SR, Krishnaswamy A. Cerebral embolic protection in transcatheter aortic valve replacement: connecting intuition and proof. JACC Cardiovasc Interv 2021;14:169-71.
117. Bagur R, Solo K, Alghofaili S, et al. Cerebral embolic protection devices during transcatheter aortic valve implantation: systematic review and meta-analysis. Stroke 2017;48:1306-15.
118. Seeger J, Gonska B, Otto M, Rottbauer W, Wöhrle J. Cerebral embolic protection during transcatheter aortic valve replacement significantly reduces death and stroke compared with unprotected procedures. JACC Cardiovasc Interv 2017;10:2297-303.
119. Testa L, Latib A, Casenghi M, Gorla R, Colombo A, Bedogni F. Cerebral Protection during transcatheter aortic valve implantation: an updated systematic review and meta-analysis. J Am Heart Assoc 2018;7:e008463.
120. Coughlan JJ, Fleck R, O’Connor C, Crean P. Mechanical thrombectomy of embolised native aortic valve post-TAVI. BMJ Case Rep 2017;2017:bcr2016218787.
121. D’Anna L, Demir O, Banerjee S, Malik I. Intravenous thrombolysis and mechanical thrombectomy in patients with stroke after TAVI: a report of two cases. J Stroke Cerebrovasc Dis 2019;28:104277.
122. Muntané-Carol G, Urena M, Munoz-Garcia A, et al. Late cerebrovascular events following transcatheter aortic valve replacement. JACC Cardiovasc Interv 2020;13:872-81.
123. Mangieri A, Montalto C, Poletti E, et al. Thrombotic versus bleeding risk after transcatheter aortic valve replacement: JACC review topic of the week. J Am Coll Cardiol 2019;74:2088-101.
124. Baumgartner H, Falk V, Bax JJ, et al. ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91.
125. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89.
126. Chakravarty T, Søndergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 2017;389:2383-92.
127. Backer O, Dangas GD, Jilaihawi H, et al; GALILEO-4D Investigators. Reduced leaflet motion after transcatheter aortic-valve replacement. N Engl J Med 2020;382:130-9.
128. Rosendael PJ, Delgado V, Bax JJ. Pacemaker implantation rate after transcatheter aortic valve implantation with early and new-generation devices: a systematic review. Eur Heart J 2018;39:2003-13.
129. Sammour Y, Krishnaswamy A, Kumar A, et al. Incidence, predictors, and implications of permanent pacemaker requirement after transcatheter aortic valve replacement. JACC Cardiovasc Interv 2021;14:115-34.
130. Auffret V, Puri R, Urena M, et al. Conduction disturbances after transcatheter aortic valve replacement: current status and future perspectives. Circulation 2017;136:1049-69.
131. Mangieri A, Lanzillo G, Bertoldi L, et al. Predictors of advanced conduction disturbances requiring a late (≥48 h) permanent pacemaker following transcatheter aortic valve replacement. JACC Cardiovasc Interv 2018;11:1519-26.
132. Mauri V, Reimann A, Stern D, et al. Predictors of permanent pacemaker implantation after transcatheter aortic valve replacement with the SAPIEN 3. JACC Cardiovasc Interv 2016;9:2200-9.
133. Latsios G, Gerckens U, Buellesfeld L, et al. “Device landing zone” calcification, assessed by MSCT, as a predictive factor for pacemaker implantation after TAVI. Catheter Cardiovasc Interv 2010;76:431-9.
134. Krishnaswamy A, Sammour Y, Mangieri A, et al. The utility of rapid atrial pacing immediately post-TAVR to predict the need for pacemaker implantation. JACC Cardiovasc Interv 2020;13:1046-54.
135. Möllmann H, Hengstenberg C, Hilker M, et al. Real-world experience using the ACURATE neo prosthesis: 30-day outcomes of 1,000 patients enrolled in the SAVI TF registry. EuroIntervention 2018;13:e1764-70.
136. Tang GHL, Zaid S, Michev I, et al. “Cusp-Overlap” view simplifies fluoroscopy-guided implantation of self-expanding valve in transcatheter aortic valve replacement. JACC Cardiovasc Interv 2018;11:1663-5.
137. Mendiz OA, Noč M, Fava CM, et al. Impact of cusp-overlap view for TAVR with self-expandable valves on 30-day conduction disturbances. J Interv Cardiol 2021;2021:9991528.
138. Sammour Y, Banerjee K, Kumar A, et al. Systematic approach to high implantation of SAPIEN-3 valve achieves a lower rate of conduction abnormalities including pacemaker implantation. Circ Cardiovasc Interv 2021;14:e009407.
139. Jilaihawi H, Zhao Z, Du R, et al. Minimizing permanent pacemaker following repositionable self-expanding transcatheter aortic valve replacement. JACC Cardiovasc Interv 2019;12:1796-807.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Khokhar AA, Ruggiero R, Chandra K, D’Agostino A, Toselli M, Mangieri A, Dudek D, Colombo A, Giannini F. Prevention and management of peri-procedural TAVR complications. Mini-invasive Surg 2022;6:2. http://dx.doi.org/10.20517/2574-1225.2021.97
AMA Style
Khokhar AA, Ruggiero R, Chandra K, D’Agostino A, Toselli M, Mangieri A, Dudek D, Colombo A, Giannini F. Prevention and management of peri-procedural TAVR complications. Mini-invasive Surgery. 2022; 6: 2. http://dx.doi.org/10.20517/2574-1225.2021.97
Chicago/Turabian Style
Khokhar, Arif A., Rossella Ruggiero, Kailash Chandra, Alessandro D’Agostino, Marco Toselli, Antonio Mangieri, Dariusz Dudek, Antonio Colombo, Francesco Giannini. 2022. "Prevention and management of peri-procedural TAVR complications" Mini-invasive Surgery. 6: 2. http://dx.doi.org/10.20517/2574-1225.2021.97
ACS Style
Khokhar, AA.; Ruggiero R.; Chandra K.; D’Agostino A.; Toselli M.; Mangieri A.; Dudek D.; Colombo A.; Giannini F. Prevention and management of peri-procedural TAVR complications. Mini-invasive. Surg. 2022, 6, 2. http://dx.doi.org/10.20517/2574-1225.2021.97
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 32 clicks
Cite This Article 32 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.