Devices, techniques, traction, suturing, and countermeasures for endoscopic submucosal dissection complications
Abstract
Endoscopic submucosal dissection (ESD) is widely performed to treat superficial colorectal tumors because it enables en bloc resection of various types of lesions. However, ESD sometimes leads to deleterious adverse events, such as perforation and delayed bleeding. Therefore, determining the precise preoperative indication for ESD is vitally important. Furthermore, small lesions with fibrosis and semi-large lesions can be managed using underwater endoscopic mucosal resection, and the “true” indication for ESD is for the treatment of larger lesions, for which ESD carries a higher risk. Here, we reviewed the devices, techniques (i.e., pocket creation method, water pressure method, and clip and flap method), traction (i.e., clip with line, pulley method, and clip with ring), suturing (i.e., line-assisted complete closure, loop clip, clip-on-clip closure method, mucosal incision around the mucosal defect, and hand-suturing), and countermeasures to address complications (i.e., bleeding after ESD, perforation, and post-ESD coagulation syndrome) that facilitate easier and safer ESD.
Keywords
INTRODUCTION
Endoscopic submucosal dissection (ESD) leads to en bloc resection of large superficial colorectal tumors which enables precise pathological diagnosis and a decrease in local recurrence compared to conventional endoscopic mucosal resection (EMR)[1]. ESD is indicated for colorectal lesions for which endoscopic en bloc resection is desired. The indications are the following: (1) lesions for which en bloc resection with snare endoscopic mucosal resection is difficult to perform [e.g., laterally spreading tumor, non-granular type, particularly pseudo-depressed type, lesions with a Vi-type pit pattern (suggesting the possibility of invasion)], carcinoma with shallow T1 (submucosal) invasion, large depressed-type tumors, and large protruded-type lesions suspected to be carcinoma; (2) mucosal tumors with submucosal fibrosis; (3) sporadic tumors in conditions of chronic inflammation such as ulcerative colitis; and (4) local residual or recurrent early carcinomas after endoscopic resection[2]. Small lesions (≤ 15 mm) with submucosal fibrosis, and small local residual or recurrent early carcinomas after endoscopic resection can be managed by underwater EMR[3-5]; therefore, ESD is primarily performed for large lesions. ESD is widely performed in Japan, other Asian countries, and several Western countries[6,7]. However, ESD sometimes results in deleterious adverse events such as perforation and delayed bleeding[8,9]. Therefore, determining precise indications for ESD preoperatively is vitally important. Here, we reviewed devices, techniques, traction, suturing, and countermeasures for complications that facilitate easier and safer ESD.
DEVICES
Endoscopy
A single-channel colonoscope with a water jet function is used to perform ESD. In some facilities, a gastroscope is also used for ESD in the distal colon because a shorter endoscope can be easily maneuvered. A colonoscope is also useful even for cases of the distal colon, because the colonoscope has a wider down angle. In the proximal colon, straightening and shortening of the endoscope are important for precise maneuverability, and single-balloon[10] or double-balloon endoscopy[11] systems are sometimes used, particularly in difficult cases.
Distal attachments
Distal attachments are very useful for performing ESD because they facilitate good visibility of the operative field and stabilize the tip of the endoscope by attaching to the mucosa or submucosa. Transparent hoods rather than black ones are preferred because they enable visibility through the hood. There are various types of hoods currently commercially available [Figure 1]. The small-caliber transparent hood (ST hood; Fujifilm, Tokyo, Japan) is particularly useful for accessing narrow spaces.
Endoknives
Various types of endoknives are commercially available. Short-needle knives, such as DualKnife (Olympus, Tokyo, Japan), HookKnife (Olympus, Tokyo, Japan), FlushKnife (Fujifilm, Tokyo, Japan), Splashneedle[12] (Pentax Medical, Tokyo, Japan), Proknife (Boston scientific Japan), and Endosaber (Sumitomo Bakelite, Tokyo, Japan) are most commonly used for colorectal ESD. These knives also possess a water-jet function that facilitates submucosal injection of saline after mucosal incision. A water-jet function is very useful for injection without changing devices, but only saline is available as high viscosity products such as hyaluronic acid are hard to pass through knives. Other types of knives, such as IT-nano (Olympus) and mucosectome (Pentax) are also used together with short-needle knives for easy and rapid submucosal dissection. Scissor-type knives, such as SB knife Jr. (Sumitomo Bakelite) and Clutch Cutter (Fujifilm) are easily used by inexperienced endoscopists[13]. A detailed explanation of endoknives is available in other sections by experienced authors.
Technique
Basically, colorectal ESD is similar to gastric ESD and comprises mucosal incision and submucosal dissection. The most distinct aspect of colorectal ESD is that maintaining elevation by submucosal injection is difficult after mucosal incision, resulting in difficulty accessing the submucosal layer during submucosal dissection. To overcome this challenge, creating a flap before the circumferential incision is crucial. Furthermore, various techniques have been developed.
Pocket creation method
Pocket creation method (PCM) was first reported by Hayashi et al.[14] in 2014. The key to successful PCM is the creation of a large submucosal pocket under the lesion using a transparent hood with a small-caliber tip. Two advantages of PCM are (1) maintaining the thick submucosal layer with a minimal mucosal incision, which prevents the leakage of injection solution; and (2) providing good traction, which facilitates efficient submucosal dissection[14]. A recent randomized controlled trial revealed that the ESD completion rate using PCM was higher than that using conventional ESD[15]. A detailed explanation of PCM is available in another section by an experienced author.
Water pressure method
This technique was originally reported as an underwater ESD[16], or saline-pocket ESD[17]. After creating the mucosal incision, the patient’s posture was changed, whereby the lesion was positioned closest to the ground to allow for water’s assistance. Next, the saline solution was infused into the dissecting submucosal layer, with active pressure applied to the submucosal layer using the water-jet function of the colonoscope[18]. Not only using the floating force provided by saline solution immersion like underwater ESD or saline-pocket ESD, active pressure to the submucosa facilitates good visibility of the submucosa, and enables efficient submucosal dissection.
A retrospective study comparing water pressure ESD and conventional ESD for tumors with submucosal fibrosis reported favorable outcomes of water pressure ESD[18]. The median procedure time was significantly shorter in the water pressure ESD group than in the conventional ESD (43.5 min vs. 72 min, P = 0.0041) with similar proportions of adverse events. Its usefulness in duodenal ESD has also been reported[19,20].
Clip flap method
Yamamoto et al.[21-23] developed a clip-flap method for mucosal creation, which is key for successful ESD. An endoclip is substituted for the mucosal flap until the flap is created completely. That is, the edge of the exfoliated mucosa is clipped with an endoclip, the distal attachment is inserted under the endoclip, and good visibility of the submucosal layer is obtained, leading to flap creation. It is particularly useful when unexpected fibrosis is encountered, or when a vertical approach is undertaken.
Traction
ESD is performed by a single endoscope (i.e., by a single hand) while most surgeries performed by surgeons, including laparoscopic surgery, are performed by two or more hands, taking advantage of traction. For colorectal ESD, gravity is the simplest method for traction, and position change is relatively easy while performing colorectal ESD, but not ESD for upper gastrointestinal lesions. In this section, we describe various methods and devices for additional traction.
Clip with line
A traction method using an endoclip attached to a string was developed for maintaining good visualization of the submucosal layer during upper gastrointestinal ESD[24,25]. However, this method was not applicable to colonic ESD because the reinsertion of the colonoscope is required to mount the endoclip attached to a string. We invented a novel traction method, called traction-assisted colorectal ESD, without reinsertion of the colonoscope[26]. By inserting a long string through the accessory channel of the colonoscope in advance, we can use the clip-with-line method after mucosal incision without reinsertion of the colonoscope. We demonstrated its efficacy (shorter procedure time and higher self-completion rate) in a randomized manner[27] and also succeeded in resecting the lesion involving a diverticulum[28].
Pulley method
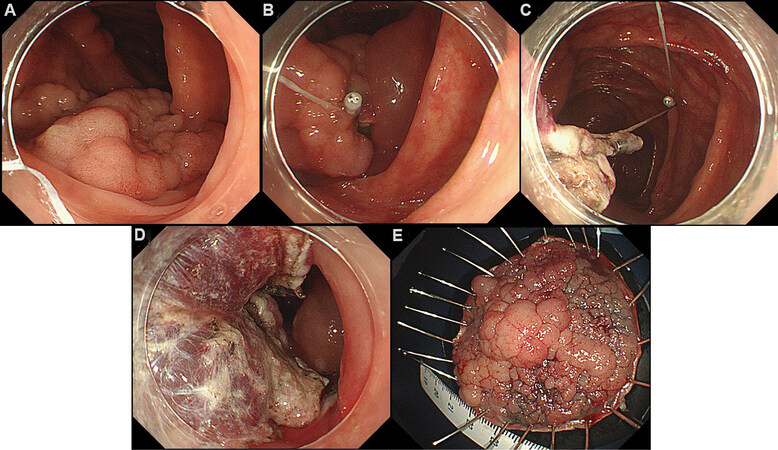
The above-mentioned traction-assisted colorectal ESD is useful in most cases, but the direction of traction is only toward the anal side, which is not always ideal. “Pulley” methods for upper gastrointestinal ESD have been reported[29-31], and we published a video case report[32] and case series[33] of “pulley” traction-assisted colorectal ESD [Figure 2].
Figure 2. (A) A 50 mm laterally spreading tumor, granular type in the ascending colon; (B) traction-assisted endoscopic submucosal dissection was performed by clip with line, but maintaining good visibility of the submucosal layer subsequently became difficult; (C) an additional clip was anchored to the opposite site of the colonic wall; (D) good visibility of the submucosal layer was obtained; (E) the lesion was resected en bloc in 100 min.
Clip with loop
Various traction methods using a loop connected to a clip, such as a clip with a ring[34], S-O clip[35-37], and loops-attached rubber band have been reported[38]. The advantage of these methods is that traction is obtained without reinsertion of the colonoscope. Furthermore, the pulley method can be used to alter the direction of traction and tighten the traction force. After resection of the lesions, a cutting loop to separate the resected lesions from the colonic wall is required.
Suturing
A large defect after colorectal ESD is associated with adverse events such as perforation and post-ESD coagulation syndrome (PECS). In previous studies, complete closure of defects after colorectal ESD may be effective in minimizing adverse events[39]; however, complete closure of large defects is often technically difficult. Here, we highlight various methods of closure because ESD ulcers are sometimes large and simple closure by clipping may be difficult.
Line-assisted complete closure
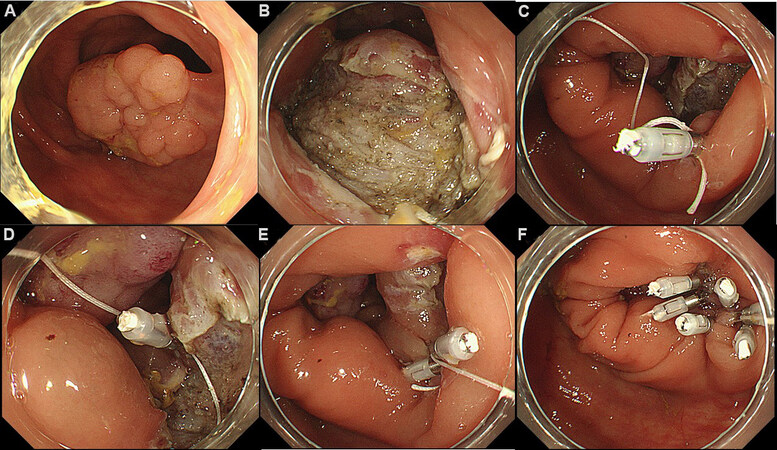
We developed a novel closure technique using a clip and line and named it line-assisted complete closure[40,41]. An endoclip with a long nylon line attached is inserted through the accessory channel and placed on the normal mucosa, just proximal to the ESD ulcer. Another endoclip without a line is anchored to the other side of the normal mucosa, and both clips are gathered by pulling the line gently through the accessory channel. Additional endoclips with/without a line are then placed until complete closure is achieved [Figure 3].
Figure 3. (A) A 40-mm laterally spreading granular-type tumor in the ascending colon; (B) mucosal defect after endoscopic submucosal dissection; (C) first clip with line is placed on the anal mucosa; (D) another clip is anchored to the oral side; (E) by pulling the line through the accessory channel, the mucosa with clips come close together; (F) additional endoclips with/without a line are placed to achieve complete closure.
Loop clip
The loop clip consists of a metal clip attached to the loop of a nylon string. The loop clip can be passed through the instrument channel of the endoscope. After ESD, a loop clip is attached to the edge of the mucosal defect around the middle of the distal side and the midpoint of the proximal side. Subsequently, regular clips are individually placed for complete closure[42].
Clip-on-clip closure method
The first clip is placed on the normal mucosa slightly away from the mucosal defect, and the second clip is placed on the handle of the first clip. Next, the teeth of the third clip pass through the gap between the teeth of the second clip, which serves as an anchor. Then, the third clip is pulled across the defect and attached to the contralateral side of the mucosal defect. Lastly, the third clip is confirmed to be securely attached to the mucosa on the opposite side of the mucosal defect. By placing additional clips, the mucosal defect is closed completely[43].
Mucosal incision around the mucosal defect
A small incision is created around the mucosal defect using the needle-type knife used for ESD. These incisions provide a better grip by conventional clips, which enables lifting the surrounding mucosa across the defect without slipping, reducing the size of the defect and easy placement of additional clips[44].
Hand-suturing
Uninterrupted endoscopic suturing of the mucosal defect after colorectal ESD with an absorbable barbed suture and a through-the-scope needle holder has been reported[45]. Although it is technically challenging and requires an extended procedure time (median: 56 min), further modification of the technique and devices could lead to clinical use.
The Overstitch System is also reportedly feasible and safe[46]. If the procedure becomes easier, widespread use is expected.
COUNTERMEASURES FOR COMPLICATIONS
Bleeding after ESD
Most bleeding following ESD occurs within 2 weeks of the procedure, particularly within the first 24 h. The rate of bleeding after ESD was reported as 0.4%-4.6%[49-55]. Bleeding is usually managed by endoscopic intervention and rarely requires transfusion or surgical intervention. Antithrombotic and anticoagulant agents have known risk factors for bleeding, and guidelines about their management for patients undergoing endoscopy have been published[56-61]. Currently, patients with comorbidities, and patients taking aspirin or warfarin have increased bleeding risk[62]. A retrospective study suggested that preventive coagulation of visible vessels in the resection area after gastric ESD may reduce the bleeding rate[63]. However, preventive coagulation of visible vessels after colorectal ESD is not common when considering the risk of delayed perforation and post-ESD coagulation syndrome because of the thinness of the colon wall. A meta-analysis of eight studies concluded that prophylactic endoscopic closure might reduce the incidence of delayed bleeding (5.2%-0.9%)[64].
Perforation
Intraprocedural perforations can usually be closed using endoclips and managed conservatively without surgical intervention when closed completely[65-67], whereas delayed perforation may lead to peritonitis and require emergency surgery. Delayed perforation usually occurs within 48 h following ESD and presents with fever and severe abdominal pain. Considering the presence of free air on computed tomography (CT) images, consultation with surgeons for the indication of surgery is mandatory. We reported that about half of the cases of delayed perforation can be managed conservatively with fasting and antibiotics, whereas the other half require emergency surgery[68]. Recently, feasibility of endoscopic closure for delayed perforation using clips, endoloop, over-the-scope clip (OTSC; Ovesco, Tübingen, Germany), and polyglycolic acid sheet has been reported[69-71].
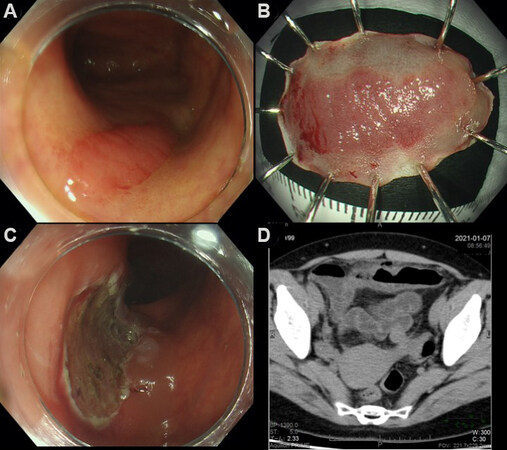
Here, we present a case of delayed perforation that was managed with OTSC. A 46-year-old woman with a laterally spreading tumor in the transverse colon was referred to our hospital. Colonoscopy at our institution revealed a 25-mm-sized non-granular type laterally spreading tumor without apparent signs of invasion [Figure 4A]. The lesion was removed en bloc by ESD in 33 min [Figure 4B] without muscle injury and prophylactic closure of the wound [Figure 4C]. The patient complained of severe abdominal pain 35 h following ESD; subsequently, a fever of 38.6 °C was recorded. CT revealed extraluminal air and increased fat concentration [Figure 4D]; therefore, delayed perforation was diagnosed, and antibiotics (tazobactam piperacillin hydrate) were initiated. The abdominal pain was localized, suggesting local peritonitis; hence, we decided to examine the ESD wound with colonoscopy using carbon dioxide insufflation.
Figure 4. (A) Laterally spreading tumor is present in the transverse colon; (B) the specimen is resected en bloc in 33 min; (C) ulcer scar just after endoscopic submucosal dissection. No muscle injury has been identified; (D) CT scan revealed extraluminal air.
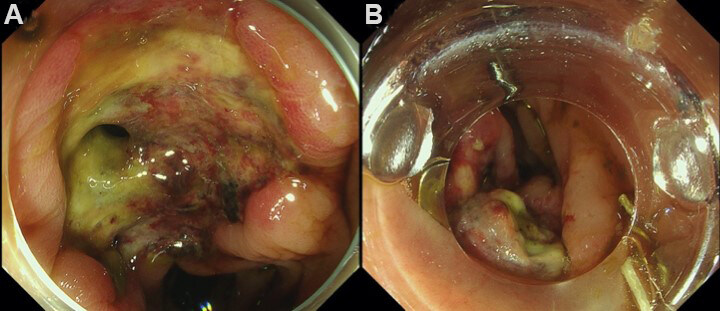
Colonoscopy 43 h after ESD revealed a 3-mm hole at the base of the ESD wound [Figure 5A]. Once we withdrew the colonoscope, after attaching an over-the-scope clip (OTSC, 11/6t) at the tip of the colonoscope, we re-approached the perforation site. Subsequently, the OTSC was placed after suctioning the perforation site and the surrounding tissue [Figure 5B]. A contrast agent was sprayed through the working channel of the colonoscope, and a CT scan showed no extraluminal leakage of the contrast medium, abscess formation, or ascites. Although laboratory tests the day after closure showed a peak C-reactive protein level of 31.2 mg/dL, her abdominal pain resolved gradually. Oral intake commenced on day 7 post-ESD, and she was discharged on day 8.
Post ESD coagulation syndrome
Even without definitive evidence of perforation on imaging, PECS sometimes occurs after colorectal ESD. Its incidence is 4.8%-40.2%[72-77], and PECS leads to deviation from the clinical path and a longer hospitalization period, although PECS can be managed conservatively with antibiotics and fasting. Known risk factors for PECS are female sex[72-74], proximal location of the lesion (cecum[73,74], cecum or ascending colon[72], and cecum to descending colon[76]), larger size[72,76], fibrosis[74], and piecemeal resection[76]. Lee et al.[77] reported that prophylactic antibiotics reduced the incidence of PECS in a single-center randomized controlled trial. The study was performed at a single center, and the sample size was relatively small; therefore, we conducted a multicenter randomized controlled trial to examine the efficacy of perioperative antibiotics for the incidence of PECS (PPAP trial, performance of perioperative antibiotics for PECS)[78]. A prospective, multicenter, randomized controlled, parallel superiority trial was completed at 21 Japanese tertiary institutions. Patients with superficial colorectal lesions ≥ 20 mm and those who were planned ESD for a single lesion were eligible. Patients with perforation during or after ESD were excluded. Before the ESD procedure, participants were randomly assigned (1:1) to either undergo conventional treatment (non-antibiotic group) or investigational treatment (antibiotic group). In the antibiotic group, ampicillin-sulbactam (3 g) was administered just before, 8 h after, and the morning after ESD. From February 5, 2019 to September 7, 2020, 432 patients were registered in total and assigned to antibiotic (n = 216) or non-antibiotic (n = 216) groups. After excluding 52 patients, 192 in the antibiotic group and 188 in the non-antibiotic group were analyzed. PECS were identified in 9 (4.7%) of the 192 patients in the antibiotic group and in 14 (7.5%) of the 188 patients in the non-antibiotic group (odds ratio = 0.61, 95% confidence interval: 0.23-1.56, P = 0.29). Therefore, we concluded that the routine administration of perioperative antibiotics for colorectal ESD is not recommended.
Several studies have reported the effect of clip closure of ESD ulcers for PECS[41,78], but the meta-analysis did not report a preventive effect for PECS[64].
Impact of underwater EMR
Small lesions (≤ 15 mm) with submucosal fibrosis, and small local residual or early recurrent carcinomas after endoscopic resection, which are difficult to be removed by conventional EMR and are indications for ESD, can be managed also by underwater EMR[3-5,79]. Furthermore, lesions sized 20-30 mm[80], and lesions slightly invading the submucosal layer (T1a) can also be managed using underwater EMR[79]. Therefore, the “true” indication for ESD is geared toward larger lesions, for which ESD harbors higher risk; this review could aid in guiding ESD for those lesions.
Full thickness resection
Endoscopic full thickness resection for gastric submucosal tumors, especially for gastrointestinal stromal tumors, is performed in several countries[81-83]. As for colon, the novel OTSC, a full-thickness resection device (FTRD, Ovesco Endoscopy, Tübingen, Germany), was first introduced in 2011[84]. Hundreds of patients were treated by FTRD, and endoscopic full-thickness resection for nonlifting, invasive lesions in the colon and rectum appears to be effective and safe[85]. Although nonlifting lesions indicated for FTRD can be removed by underwater EMR, invasive lesions, especially invasive to deeper layers, such as muscle layers are almost impossible to be removed by EMR or ESD.
Future trends
ESD using a CO2 laser has been reported in vivo and ex vivo (porcine colon tissue)[86]. Because saline or sodium hyaluronate solution injected into the submucosal layer during ESD procedure has a high absorption coefficient at the wavelength of the CO2 laser, safer ESD is expected with a CO2 laser with less thermal damage to tissue.
An endoscopic device for colonic submucosal dissection using a combination of bipolar radiofrequency and microwave modalities has also been reported in a porcine model[87]. Currently, ESD can be performed only by experienced endoscopists, but with the advancement in devices and techniques, more widespread use could be facilitated.
Robot-assisted ESD is also attempted in a porcine model[88,89]. With two hands available like surgery, more efficient resection can be achieved. Shorter procedure time and less complication are reported by ESD novices, and robot-assisted ESD is also associated with lower physical and mental workloads.
CONCLUSION
With the development of various instruments and techniques, ESD has become more common and safer. However, because ESD sometimes leads to deleterious complications, the indications for ESD should be carefully considered.
DECLARATIONS
Authors’ contributionsDrafted the manuscript: Shichijo S
Critical revision and final approval of the article: Takeuchi Y
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestSatoki Shichijo has received honoraria for his lectures from Olympus, Boston Scientific Japan, Daiichi-Sankyo, EA Pharma, Zeria Pharmaceutical, The Japanese Society of Gastroenterology, and Japan Gastroenterological Endoscopy Society. Yoji Takeuchi has received honoraria for his lectures from Olympus, Boston Scientific Japan, Daiichi-Sankyo, Miyarisan Pharmaceutical, Asuka Pharmaceutical, AstraZeneca, EA Pharma, Zeria Pharmaceutical, Fujifilm, Kaneka Medix, Kyorin Pharmaceutical, and Japan Gastroenterological Endoscopy Society.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Oka S, Tanaka S, Saito Y, et al. Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015;110:697-707.
2. Tanaka S, Kashida H, Saito Y, et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2020;32:219-39.
3. Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc 2012;75:1086-91.
4. Shichijo S, Takeuchi Y, Uedo N, Ishihara R. Management of local recurrence after endoscopic resection of neoplastic colonic polyps. World J Gastrointest Endosc 2018;10:378-82.
5. Ohmori M, Yamasaki Y, Iwagami H, et al. Propensity score-matched analysis of endoscopic resection for recurrent colorectal neoplasms: a pilot study. J Gastroenterol Hepatol 2021;36:2568-74.
6. Zhang X, Ly EK, Nithyanand S, et al. Learning curve for endoscopic submucosal dissection with an untutored, prevalence-based approach in the United States. Clin Gastroenterol Hepatol 2020;18:580-8.e1.
7. Rönnow C, Uedo N, Toth E, Thorlacius H. Endoscopic submucosal dissection of 301 large colorectal neoplasias: outcome and learning curve from a specialized center in Europe. Endosc Int Open 2018;06:E1340-8.
8. De Ceglie A, Hassan C, Mangiavillano B, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: a systematic review. Crit Rev Oncol Hematol 2016;104:138-55.
9. Takeuchi Y, Iishi H, Tanaka S, et al. Factors associated with technical difficulties and adverse events of colorectal endoscopic submucosal dissection: retrospective exploratory factor analysis of a multicenter prospective cohort. Int J Colorectal Dis 2014;29:1275-84.
10. Ohya T, Ohata K, Sumiyama K, et al. Balloon overtube-guided colorectal endoscopic submucosal dissection. World J Gastroenterol 2009;15:6086-90.
11. Yamashina T, Hayashi Y, Sakamoto H, et al. Balloon-assisted endoscopy facilitates endoscopic submucosal dissection of difficult superficial proximal colon tumors. Endoscopy 2018;50:800-8.
12. Sakaguchi Y, Tsuji Y, Fujishiro M, et al. Evaluation of endoscopic submucosal dissection using a new endosurgical knife DN-D2718B: a first clinical feasibility study. Endosc Int Open 2017;5:E670-4.
13. Yamashina T, Takeuchi Y, Nagai K, et al. Scissor-type knife significantly improves self-completion rate of colorectal endoscopic submucosal dissection: single-center prospective randomized trial. Dig Endosc 2017;29:322-9.
14. Hayashi Y, Sunada K, Takahashi H, et al. Pocket-creation method of endoscopic submucosal dissection to achieve en bloc resection of giant colorectal subpedunculated neoplastic lesions. Endoscopy 2014;46 Suppl 1 UCTN:E421-2.
15. Yamashina T, Nemoto D, Hayashi Y, et al. Prospective randomized trial comparing the pocket-creation method and conventional method of colorectal endoscopic submucosal dissection. Gastrointest Endosc 2020;92:368-79.
16. Akasaka T, Takeuchi Y, Uedo N, Ishihara R, Iishi H. “Underwater” endoscopic submucosal dissection for superficial esophageal neoplasms. Gastrointest Endosc 2017;85:251-2.
17. Harada H, Nakahara R, Murakami D, et al. Saline-pocket endoscopic submucosal dissection for superficial colorectal neoplasms: a randomized controlled trial (with video). Gastrointest Endosc 2019;90:278-87.
18. Ozeki Y, Hirasawa K, Ikeda R, et al. Safety and efficacy of water pressure endoscopic submucosal dissection for colorectal tumors with submucosal fibrosis (with video). Gastrointest Endosc 2021;94:607-17.e2.
19. Yahagi N, Nishizawa T, Sasaki M, Ochiai Y, Uraoka T. Water pressure method for duodenal endoscopic submucosal dissection. Endoscopy 2017;49:E227-8.
20. Kato M, Takatori Y, Sasaki M, et al. Water pressure method for duodenal endoscopic submucosal dissection (with video). Gastrointest Endosc 2021;93:942-9.
21. Yamamoto K, Hayashi S, Nakabori T, Shibuya M, Ichiba M, Inada M. Endoscopic submucosal dissection using endoclips to assist in mucosal flap formation (novel technique: “clip flap method”). Endoscopy 2012;44 Suppl 2 UCTN:E334-5.
22. Yamamoto K, Hayashi S, Saiki H, et al. Endoscopic submucosal dissection for large superficial colorectal tumors using the “clip-flap method”. Endoscopy 2015;47:262-5.
23. Yamamoto K, Hayashi S, Nishida T, et al. Effective use of the “clip-flap” method for the endoscopic submucosal dissection of a difficult-to-approach superficial gastric tumor. Endoscopy 2015;47 Suppl 1 UCTN:E318-9.
24. Yoshida M, Takizawa K, Suzuki S, et al. CONNECT-G Study Group. Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: a multicenter, randomized controlled trial (with video). Gastrointest Endosc 2018;87:1231-40.
25. Yoshida M, Takizawa K, Nonaka S, et al. CONNECT-E Study Group. Conventional versus traction-assisted endoscopic submucosal dissection for large esophageal cancers: a multicenter, randomized controlled trial (with video). Gastrointest Endosc 2020;91:55-65.e2.
26. Yamasaki Y, Takeuchi Y, Hanaoka N, et al. A novel traction method using an endoclip attached to a nylon string during colonic endoscopic submucosal dissection. Endoscopy 2015;47 Suppl 1 UCTN:E238-9.
27. Yamasaki Y, Takeuchi Y, Uedo N, et al. Efficacy of traction-assisted colorectal endoscopic submucosal dissection using a clip-and-thread technique: a prospective randomized study. Dig Endosc 2018;30:467-76.
28. Shichijo S, Yamasaki Y, Takeuchi Y. Case of colonic adenoma involving a diverticulum resected by a traction-assisted endoscopic submucosal dissection technique. Dig Endosc 2017;29:729-30.
29. Rieder E, Makris KI, Martinec DV, Swanström LL. The suture-pulley method for endolumenal triangulation in endoscopic submucosal dissection. Endoscopy 2011;43 Suppl 2 UCTN:E319-20.
30. Aihara H, Kumar N, Ryou M, Abidi W, Ryan MB, Thompson CC. Facilitating endoscopic submucosal dissection: the suture-pulley method significantly improves procedure time and minimizes technical difficulty compared with conventional technique: an ex vivo study (with video). Gastrointest Endosc 2014;80:495-502.
31. Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc 2012;45:375-8.
32. Shichijo S, Matsuno K, Takeuchi Y, Uedo N, Ishihara R. Pulley traction-assisted colonic endoscopic submucosal dissection affords good visibility of submucosal layer. VideoGIE 2018;3:358-60.
33. Shichijo S, Takeuchi Y, Matsuno K, et al. Pulley traction-assisted colonic endoscopic submucosal dissection: a retrospective case series. Dig Dis 2019;37:473-7.
34. Mori H, Kobara H, Nishiyama N, Fujihara S, Matsunaga T, Masaki T. Novel effective and repeatedly available ring-thread counter traction for safer colorectal endoscopic submucosal dissection. Surg Endosc 2017;31:3040-7.
35. Ritsuno H, Sakamoto N, Osada T, et al. Prospective clinical trial of traction device-assisted endoscopic submucosal dissection of large superficial colorectal tumors using the S-O clip. Surg Endosc 2014;28:3143-9.
36. Sakamoto N, Osada T, Shibuya T, et al. The facilitation of a new traction device (S-O clip) assisting endoscopic submucosal dissection for superficial colorectal neoplasms. Endoscopy 2008;40 Suppl 2:E94-5.
37. Sakamoto N, Osada T, Shibuya T, et al. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc 2009;69:1370-4.
38. Osada T, Sakamoto N, Shibuya T, et al. “Loops-attached rubber band” facilitation of endoscopic submucosal dissection of superficial colorectal neoplasm. Endoscopy 2008;40 Suppl 2:E101-2.
39. Harada H, Suehiro S, Murakami D, et al. Clinical impact of prophylactic clip closure of mucosal defects after colorectal endoscopic submucosal dissection. Endosc Int Open 2017;5:E1165-71.
40. Kato M, Takeuchi Y, Yamasaki Y, et al. Technical feasibility of line-assisted complete closure technique for large mucosal defects after colorectal endoscopic submucosal dissection. Endosc Int Open 2017;5:E11-6.
41. Yamasaki Y, Takeuchi Y, Iwatsubo T, et al. Line-assisted complete closure for a large mucosal defect after colorectal endoscopic submucosal dissection decreased post-electrocoagulation syndrome. Dig Endosc 2018;30:633-41.
42. Sakamoto N, Beppu K, Matsumoto K, et al. “Loop Clip”, a new closure device for large mucosal defects after EMR and ESD. Endoscopy 2008;40 Suppl 2:E97-8.
43. Nomura T, Matsuzaki I, Sugimoto S, Oyamda J, Kamei A, Kobayashi M. Clip-on-clip closure method for a mucosal defect after colorectal endoscopic submucosal dissection: a prospective feasibility study. Surg Endosc 2020;34:1412-6.
44. Otake Y, Saito Y, Sakamoto T, et al. New closure technique for large mucosal defects after endoscopic submucosal dissection of colorectal tumors (with video). Gastrointest Endosc 2012;75:663-7.
45. Abe S, Saito Y, Tanaka Y, et al. A novel endoscopic hand-suturing technique for defect closure after colorectal endoscopic submucosal dissection: a pilot study. Endoscopy 2020;52:780-5.
46. Kantsevoy SV, Bitner M, Mitrakov AA, Thuluvath PJ. Endoscopic suturing closure of large mucosal defects after endoscopic submucosal dissection is technically feasible, fast, and eliminates the need for hospitalization (with videos). Gastrointest Endosc 2014;79:503-7.
47. Yamashina T, Uedo N, Akasaka T, et al. Comparison of underwater vs conventional endoscopic mucosal resection of intermediate-size colorectal polyps. Gastroenterology 2019;157:451-61.e2.
48. Yamasaki Y, Harada K, Oka S, et al. Feasibility of underwater clip closure for large mucosal defects after colorectal endoscopic submucosal dissection. Digestion 2019;99:327-32.
49. Takeuchi Y, Ohta T, Matsui F, Nagai K, Uedo N. Indication, strategy and outcomes of endoscopic submucosal dissection for colorectal neoplasm. Dig Endosc 2012;24 Suppl 1:100-4.
50. Niimi K, Fujishiro M, Kodashima S, et al. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 2010;42:723-9.
51. Oka S, Tanaka S, Kanao H, et al. Current status in the occurrence of postoperative bleeding, perforation and residual/local recurrence during colonoscopic treatment in Japan. Dig Endosc 2010;22:376-80.
52. Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010;72:1217-25.
53. Toyonaga T, Nishino E, Man-I M, East JE, Azuma T. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc 2012;45:362-74.
54. Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013;27:3262-70.
55. Lee EJ, Lee JB, Lee SH, et al. Endoscopic submucosal dissection for colorectal tumors--1,000 colorectal ESD cases: one specialized institute’s experiences. Surg Endosc 2013;27:31-9.
56. Veitch AM, Vanbiervliet G, Gershlick AH, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut 2016;65:374-89.
57. Acosta RD, Abraham NS, Chandrasekhara V, et al. ASGE Standards of Practice Committee. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc 2016;83:3-16.
58. Chan FKL, Goh KL, Reddy N, et al. Management of patients on antithrombotic agents undergoing emergency and elective endoscopy: joint Asian Pacific Association of Gastroenterology (APAGE) and Asian Pacific Society for Digestive Endoscopy (APSDE) practice guidelines. Gut 2018;67:405-17.
59. Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:299S-339S.
60. Kato M, Uedo N, Hokimoto S, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment: 2017 appendix on anticoagulants including direct oral anticoagulants. Dig Endosc 2018;30:433-40.
61. Fujimoto K, Fujishiro M, Kato M, et al. Japan Gastroenterological Endoscopy Society. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc 2014;26:1-14.
62. Rechenmacher SJ, Fang JC. Bridging anticoagulation: primum non nocere. J Am Coll Cardiol 2015;66:1392-403.
63. Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy 2008;40:179-83.
64. Liu M, Zhang Y, Wang Y, Zhu H, Xu H. Effect of prophylactic closure on adverse events after colorectal endoscopic submucosal dissection: a meta-analysis. J Gastroenterol Hepatol 2020;35:1869-77.
65. Taku K, Sano Y, Fu KI, et al. Iatrogenic perforation associated with therapeutic colonoscopy: a multicenter study in Japan. J Gastroenterol Hepatol 2007;22:1409-14.
66. Repici A, Pellicano R, Strangio G, Danese S, Fagoonee S, Malesci A. Endoscopic mucosal resection for early colorectal neoplasia: pathologic basis, procedures, and outcomes. Dis Colon Rectum 2009;52:1502-15.
67. Nakahira H, Takeuchi Y, Garcia JS, et al. Line-assisted endoscopic complete closure of a large perforation during colonic endoscopic submucosal dissection. Endoscopy 2018;50:E32-3.
68. Iwatsubo T, Takeuchi Y, Yamasaki Y, et al. Differences in clinical course of intraprocedural and delayed perforation caused by endoscopic submucosal dissection for colorectal neoplasms: a retrospective study. Dig Dis 2019;37:53-62.
69. Xiao YF, Bai JY, Yu J, et al. Endoscopic treatment of delayed colon perforation: the enteroscopy overtube approach. Endoscopy 2014;46:503-8.
70. Kuwabara H, Chiba H, Tachikawa J, Okada N, Arimoto J, Nakaoka M. Endoscopic closure using over-the-scope clip for delayed colonic perforation after hybrid endoscopic submucosal dissection. Endoscopy 2020;52:E368-9.
71. Nagami Y, Fukunaga S, Kanamori A, et al. Endoscopic closure using polyglycolic acid sheets for delayed perforation after colonic endoscopic submucosal dissection. Endoscopy 2020;52:E11-2.
72. Yamashina T, Takeuchi Y, Uedo N, et al. Features of electrocoagulation syndrome after endoscopic submucosal dissection for colorectal neoplasm. J Gastroenterol Hepatol 2016;31:615-20.
73. Arimoto J, Higurashi T, Kato S, et al. Risk factors for post-colorectal endoscopic submucosal dissection (ESD) coagulation syndrome: a multicenter, prospective, observational study. Endosc Int Open 2018;6:E342-9.
74. Ito S, Hotta K, Imai K, et al. Risk factors of post-endoscopic submucosal dissection electrocoagulation syndrome for colorectal neoplasm. J Gastroenterol Hepatol 2018;33:2001-6.
75. Hong MJ, Kim JH, Lee SY, Sung IK, Park HS, Shim CS. Prevalence and clinical features of coagulation syndrome after endoscopic submucosal dissection for colorectal neoplasms. Dig Dis Sci 2015;60:211-6.
76. Jung D, Youn YH, Jahng J, Kim JH, Park H. Risk of electrocoagulation syndrome after endoscopic submucosal dissection in the colon and rectum. Endoscopy 2013;45:714-7.
77. Lee SP, Sung IK, Kim JH, et al. A randomized controlled trial of prophylactic antibiotics in the prevention of electrocoagulation syndrome after colorectal endoscopic submucosal dissection. Gastrointest Endosc 2017;86:349-57.e2.
78. Shichijo S, Takeuchi Y, Shimodate Y, et al. Kansai Endoscopic Device Selection Conference in Kansai Research Group. Performance of perioperative antibiotics against post-endoscopic submucosal dissection coagulation syndrome: a multicenter randomized controlled trial. Gastrointest Endosc 2022;95:349-59.
79. Fukuda H, Takeuchi Y, Shoji A, et al. Curative value of underwater endoscopic mucosal resection for submucosally invasive colorectal cancer. J Gastroenterol Hepatol 2021;36:2471-8.
80. Inoue T, Nakagawa K, Yamasaki Y, et al. Underwater endoscopic mucosal resection versus endoscopic submucosal dissection for 20-30 mm colorectal polyps. J Gastroenterol Hepatol 2021;36:2549-57.
81. Zhou PH, Yao LQ, Qin XY, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 2011;25:2926-31.
82. Yu C, Liao G, Fan C, et al. Long-term outcomes of endoscopic resection of gastric GISTs. Surg Endosc 2017;31:4799-804.
83. Shichijo S, Uedo N, Yanagimoto Y, et al. Endoscopic full-thickness resection of gastric gastrointestinal stromal tumor: a Japanese case series. Ann Gastroenterol 2019;32:593-9.
84. Andrisani G, Pizzicannella M, Martino M, et al. Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: a single-centre study. Dig Liver Dis 2017;49:1009-13.
85. Li P, Ma B, Gong S, Zhang X, Li W. Efficacy and safety of endoscopic full-thickness resection in the colon and rectum using an over-the-scope device: a meta-analysis. Surg Endosc 2021;35:249-59.
86. Noguchi T, Hazama H, Nishimura T, Morita Y, Awazu K. Enhancement of the safety and efficacy of colorectal endoscopic submucosal dissection using a CO2 laser. Lasers Med Sci 2020;35:421-7.
87. Tsiamoulos ZP, Sibbons P, Morris S, Hancock CP, Saunders BP. A novel multimodality endoscopic device for colonic submucosal dissection using a combination of bipolar radiofrequency and microwave modalities. Endoscopy 2016;48:271-6.
88. Chiu PWY, Ho KY, Phee SJ. Colonic endoscopic submucosal dissection using a novel robotic system (with video). Gastrointest Endosc 2021;93:1172-7.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Shichijo S, Takeuchi Y. Devices, techniques, traction, suturing, and countermeasures for endoscopic submucosal dissection complications. Mini-invasive Surg 2022;6:19. http://dx.doi.org/10.20517/2574-1225.2021.121
AMA Style
Shichijo S, Takeuchi Y. Devices, techniques, traction, suturing, and countermeasures for endoscopic submucosal dissection complications. Mini-invasive Surgery. 2022; 6: 19. http://dx.doi.org/10.20517/2574-1225.2021.121
Chicago/Turabian Style
Shichijo, Satoki, Yoji Takeuchi. 2022. "Devices, techniques, traction, suturing, and countermeasures for endoscopic submucosal dissection complications" Mini-invasive Surgery. 6: 19. http://dx.doi.org/10.20517/2574-1225.2021.121
ACS Style
Shichijo, S.; Takeuchi Y. Devices, techniques, traction, suturing, and countermeasures for endoscopic submucosal dissection complications. Mini-invasive. Surg. 2022, 6, 19. http://dx.doi.org/10.20517/2574-1225.2021.121
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 10 clicks
Cite This Article 10 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.