Endoscopic management of early complications following bariatric surgery
Abstract
Bariatric surgery procedures are increasing exponentially with the obesity epidemic. Early complications are defined as those that occur within the first 30 days after surgery. Some of the most common early complications are leaks, bleeding, stricture or stenosis and bezoar, all of which can be diagnosed and treated endoscopically. Upper endoscopy has been proven to be safe in the early postoperative period and different endoscopic modalities, like stenting, clipping, overstitch, among others, are part of the armamentarium the endoscopist should have available to address complications and potentially avoid the morbidity and mortality associated with re-operation.
Keywords
INTRODUCTION
The obesity epidemic in the United States continues to grow and become an increasing public health problem[1,2]. As a result, the number of bariatric surgeries performed also has increased[1]. Weight loss surgery improves the chronic diseases that are associated with obesity and reduces the relative risk of death[1-11]. Nonetheless, the morbidity rate of surgery is between 4% and 10% with complications presenting most commonly within the first 30 days after surgery[6].
Early major complications (< 1.6%) that could lead to death include anastomotic leak, pulmonary embolism (PE) and myocardial infarction (MI)[7]. According to data from the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program Database Registry (MBSAQIP) between July 2020 through June 2021, complications seen within 30 days include bleeding requiring transfusion (0.72%), GI tract bleeding (0.38%), bowel obstruction (0.34%), urinary tract infections (0.31%), anastomotic or staple line leak (0.23%), venous thromboembolism (0.22%) and organ space infection (0.06%)[7,8,12]. Of patients requiring intervention within 30 days after surgery, the most common are due to anastomotic or staple line leak, gastrointestinal tract bleeding, and for a GI tract stricture or obstruction. Leaks and bleeding have a higher impact on end-organ dysfunction and may require urgent reoperations or intensive care unit admissions, while other less lethal early intraluminal complications, such as bezoar, stricture and stenosis, can be managed with decreased urgency[2-8]. All of these events could be encountered after any bariatric procedure and depending on the weight loss surgery performed; there are other possible procedure-specific complications[6]. The most commonly performed procedures are the laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass (RYGB), but other weight loss surgeries that are increasing in popularity are the duodenal switch (DS) and the single anastomosis duodenal ileal bypass[2-8]. Late complications (> 30 days) include gastroesophageal reflux disease, strictures, internal or incisional hernias[2-12].
Esophagogastroduodenoscopy (EGD) is the cornerstone for the diagnosis and treatment of intraluminal complications after bariatric surgery[13]. Evidence has shown that EGD is a safe and cost-effective procedure in the early postoperative period that should not be delayed[13]. Advances in surgical and interventional endoscopy have provided new, less invasive and effective options to treat intraluminal complications[2,5-11,13]. It is associated with a lower morbidity rate compared to revisional surgery, and it can be adopted either as a definitive treatment, or as a bridge for revisional surgery[6-11,13].
COMPLICATIONS
Leak
Staple line or anastomotic leaks are rare, but they can lead to significant morbidity and mortality[6-8,13]. Leaks are one of the most dreaded complications after bariatric surgery since they increase hospital stay and increase the risk of fistula formation (gastroenteric or gastrobronchial) which can take months to heal[2-8]. Higher risk patients include those undergoing revisional surgery, patients with a BMI > 50 kg/m2, or patients with dysmetabolic syndrome X[2-8]. The causes of leaks are unclear, but hypotheses include technical factors like anastomotic tension, tissue ischemia, insufficient staple height, overlapping staple line, tissue thickness and blood supply[14,15]. While leak rates after bariatric surgery are very low at MBSAQIP centers, it has been reported as high as 1%-9%, although rates differ depending on the specific procedure[4,5,7,8].
After LSG, leaks are most likely to occur near, or at, the Angle of His, where the staple line is within proximity to the gastroesophageal junction[2,9]. It can occur due to distal stenosis at the incisura, increased proximal pressure, thinner tissue, and a relative watershed area at the Angle of His seen in angiography studies[2,5,9]. Inadvertent dog-ears created at the proximal sleeve can be affected by this watershed area and the tip can become ischemic and slough. Leaks after a LSG have been reported to occur in 1%-9% of cases[2,9,10]. After RYGB, the reported leak rate is between 0.09%-1.14%, and they can occur at the gastrojejunal (GJ) anastomosis, jejunojejunal (JJ) anastomosis or any staple line in the GI tract[2,11]. The GJ anastomosis is the most susceptible due to the single blood supply to the gastric pouch[2,10]. Leaks after a DS are most likely to occur at the duodenoileal anastomosis.
A leak should be suspected if the patient develops abdominal pain, dyspnea, tachycardia, and fevers, approximately 1 to 14 days after the operation[2,6]. Early recognition and an appropriate work-up should be performed to assess the location and size of the defect and to initiate prompt intervention. Antibiotics should be started for infection control and nutritional optimization (enteral feedings through a feeding tube distal to the leak if tolerated, or parenteral nutrition) should be instituted which will aid in the healing process[2,6]. Clinical stability of the patient will guide further management.
Hemodynamically unstable patients who present with hypotension and sustained tachycardia (> 120 bpm) should be resuscitated and assessed for a PE or MI[2]. Emergency surgery should follow if those are ruled out. Laparoscopic exploration can be performed if the patient can tolerate it and the expertise of the surgeon allows[2,5,6]. The goals of surgery are to wash out any spillage and contamination, control the leak with drainage, debridement of the leak site with closure, if possible, and establish feeding access[2,5,9]. Closure of the leak site, if identified, can be done with interrupted sutures and an omental patch which can provide protection to the repair. Closure of the leak is less successful after postoperative day 3 from the primary procedure due to poor healing, extensive inflammation and necrosis[2-5]. Immediate surgical management of leaks is associated with increased morbidity and mortality and is reserved for patients with clear indications (hemodynamically unstable, septic shock, diffuse peritonitis). Alternative treatment options explored can be for the clinically stable patient[2-5].
If the patient is hemodynamically stable, further workup is needed to assess for other causes of tachycardia, including bleeding, hypovolemia, pneumonia[2-5]. A CT scan with oral contrast is needed for the workup of a leak[2-6]. This diagnostic study will also help in assessing for other differential diagnoses. Oral contrast given prior to the scan is imperative to be able to diagnose a proximal leak. The detection rate of a GJ anastomosis or LSG leak using a CT scan is reported as 60%-80%[2-8]. Upper gastrointestinal series (UGI) has less sensitivity in the diagnosis of a proximal leak[3,4,6-8]. Unexplained persistent symptoms and signs without radiographic evidence of any type of leak should prompt early surgical exploration[7,8]. CT evidence of abdominal fluid collection, phlegmon, or abscess is indicative of a leak, even if no extravasation of contrast is seen. In this case, the fluid collection will need to be drained by interventional radiology, laparoscopically, or transluminal endoscopic debridement and drainage (by endo-vacuum or with pigtail catheter)[6-8].
For hemodynamically stable patients, non-surgical options should be considered before resorting to surgery[3-8]. Waiting at least 6 months before surgical treatment is advised, since at this point the probability of healing with endoscopic treatment significantly decreases (from 76.4% at 1 month to 48.5% after 6 months)[3-8]. Endoscopic treatment success markedly decreases with time; therefore, early diagnosis is imperative[8]. Predictors of endoscopic treatment success include early leak diagnosis (< 3 postoperative days), early endoscopic treatment (< 21 days from leak diagnosis), leak size (< 1 cm) and absence of a history of gastric banding[3-8].
Endoluminal pressure varies depending on the bariatric procedure performed[7,8]. The gastric pouch created during a RYGB is a low-pressure system, for which surgical or endoscopic strategies to control the leak but not close or repair the perforation site are more likely to be effective (72%) in comparison to LSG leak[7,8]. LSG are considered a high-pressure system and hence leaks are more difficult to treat. The presence of the pylorus and lower esophageal sphincter creates the high-pressure, as does the presence of a stenosis, twist or kink[5,7-11]. These issues need to be addressed in a timely manner to successfully treat the leak. Endoscopic techniques used to allow better drainage of the sleeve and reduce intraluminal pressure include Botox injections and pneumatic balloon dilation (achalasia balloon) at the pylorus.
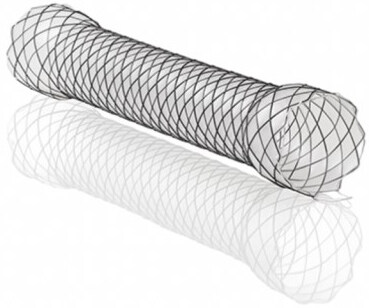
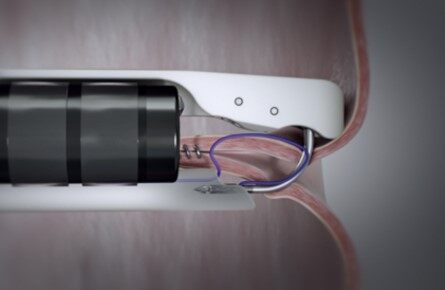
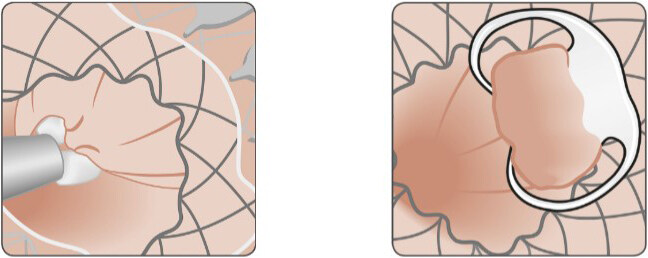
The key goals of endoscopic treatment are to cover/exclude the wall defect (self-expandible metallic stents - SEMS) [Figure 1] or close the perforation site clips [Figures 2 and 3], endoscopic suturing (Overstitch) [Figure 4], and allow healing by secondary intention with the aid of a vacuum or septotomy)[6] [Table 1]. Patients who have leaks for more than 30 days can develop into fistulas if they communicate with another epithelialized surface (the GI tract and another organ or surface). Fistulas are defined as the abnormal communication between 2 epithelialized surfaces. Chronic leaks can be treated with clips, stents or vacuum dressings[6-11,13-16].
Figure 1. Boston Scientific esophageal stent system[28].
Figure 2. Boston Scientific resolution clips[28].
Figure 3. Ovesco clip[29].
Figure 4. Overstitch endoscopic suturing system[30].
Treatment of leak
| Cover/exclude wall defect | Self-expandible metallic stents (SEMS) [Figure 1] |
| Close perforation site | Through the scope clipping (TTSC) [Figure 2] |
| Over the scope clips (OTSC - Ovesco) [Figure 3] | |
| Tissue adhesives and plugs: Fibrin or cyanoacrylate sealants | |
| Overstitch endoscopic suturing system [Figure 4] | |
| Drainage/debridement | Debridement with snare, net or basket |
| Nasocystic catheter | |
| Endoscopic internal drainage: double-pigtails catheters [Figure 8] | |
| Endoscopic vacuum assisted closure (EVAC) [Figure 9] | |
| Septotomy |
SEMS are the most common modality used to treat leaks[6] [Figure 1]. The stent is placed over the perforation, excluding and isolating the wall defect from the esophageal and gastric secretions. This prevents further intra-abdominal contamination and permits the patient to resume oral intake[6,14-16]. Uncovered SEMS are not used for non-malignant diseases for their high risk of bleeding, recurrent stricture, erosion and tissue embedment making their removal difficult. Therefore, covered (fully or partially) stents are used for the treatment of bariatric surgery leaks[2,5,9]. Stents are placed under fluoroscopy and in LSG should ideally cover from the lower esophageal sphincter through the pylorus to reduce pressure and allow the leak to heal[17-19]. To prevent stent incorporation into the native tissue, they are removed at 2-3 weeks and a leak study with contrast is obtained to assess healing and develop further treatment plans. Side effects of stents are dysphagia, reflux symptoms and stent migration[6,15-19]. Medical therapy with antispasmodics and antiemetics can be used to treat the symptoms and help patients tolerate the stent for maximal time and oral intake. Stent migration is described in 40% of cases and modalities like clips and suturing have been used to minimize migration with some success[6,11,13-18]. Successful leak closure with stents has been described to be up to 87.88%[18-20]. Endoscopic suturing, OTSCs, and glue injection have been used in adjuncts to stenting[17-19] [Figures 3, 5, 6].
Figure 5. Scheme of distal fixation of the stent with Ovesco clip[29].
Figure 6. Distal fixation of a stent with Ovesco clip. Ex-vivo model[29].
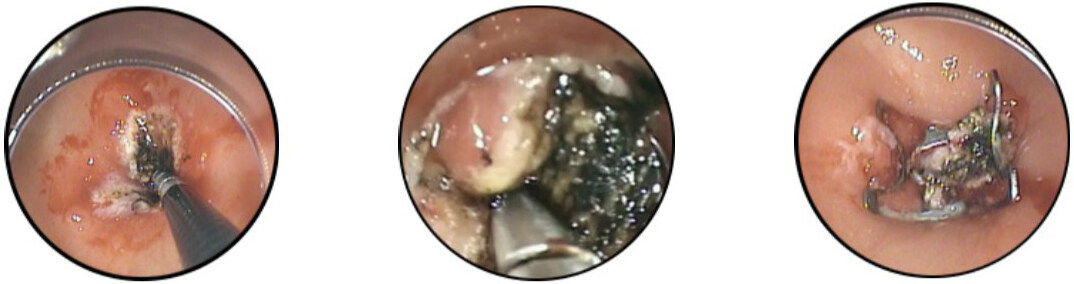
Attempting wall closure can be done if the defect is small and depending on the endoscopist’s expertise. The scope clipping (TTSC) is usually used in defects less than 1 cm in size[9,18] [Figure 2]. The edges are approximated, and the clip is deployed into the grasped tissue. Multiple clips can be placed in parallel to warrant the closure of the defect. If clips dislodge or the tissue is too friable or necrotic that the grasp of the edges was inadequate, treatment might need to be repeated[16-19]. Over the scope clips (OTSC - Ovesco) can cover a larger defect, up to 30 mm in size, and achieve a full thickness closure [Figure 3][19,21]. Its success is reported in 70%-100% of LSG leaks[17-19]. Suction is used to pull adequate tissue and appropriately approximate edges [Figure 7]. Both TTSC and OTSC are more successful if prior abscess drainage is performed[6,16-19]. Combining OTSC with other endoscopic therapies provides a higher probability of success [Figure 6]. Tissue adhesives, like fibrin or cyanoacrylate sealants, and plugs are also used to treat leaks. Multiple sessions are required where the sealant is injected into the lumen of small defects[17-19]. If the defect is large the sealant might migrate before coagulating. These tissue adhesives and plugs are mostly used for closure of fistula tracts with a success rate of 80%[19]. Lastly, endoscopic suturing (Overstitch) can also be used for closure of the wall defect when the defect size is large and other methods might not be successful or have failed [Figure 4]. Data on usage of the endoscopic suturing device is lacking, but early case reports show promising results[18,19].
Figure 7. Closure of persistent fistula using OTSC. Source: Dr. Thomas Kratt, Interventional Endoscopy, Klinik für Allgemeine, Viszeral-und Transplantationschirurgie, University Hospital Tuebingen, Germany.
Patients with lumen-adjacent fluid collections that are not percutaneously drained by interventional radiology, or by surgery, are candidates for endoscopic debridement and drainage. Transgastric stents have been reported to be 90% efficient in the treatment of acute or early leaks[20,22]. Simple fluid collections do not need to be debrided; they only need drainage. The presence of foreign material, like sutures, needs to be assessed and removal is necessary to achieve proper healing[6,19,20,22]. Complex fluid collections with necrotic tissue and/or septations need to be debrided (endoscopically or surgically) before drainage[22,23]. Endoscopic debridement can be done using a snare, net or basket[5,20,21]. Different approaches to drainages have been described. Nasocystic catheters are placed in large cavities that need flushing or irrigation to aid drainage. Endoscopic internal drainage (EID) refers to the use of double-pigtail catheters, which keep the tract between the stomach lumen and the infected cavity open[20,22] [Figure 8]. This catheter provides continuous outflow from the cavity, which will progress into a “virtual” space where only the tip of the catheter is located[20,22]. Removal of the catheter is performed as soon as clinical and radiological resolution is seen. The clinical success of EID ranges from 70%-85%[20,22].
Figure 8. Double-pigtail catheter[30].
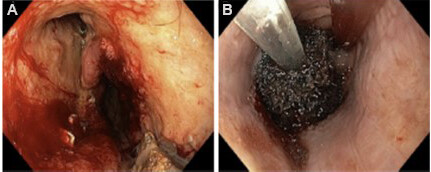
Endoscopic vacuum-assisted closure (EVAC) can be used as first line or as salvage therapy after other endoscopic treatments have failed[5,6,9,16] [Figure 9]. It consists of endoscopically inserting a sponge into the cavity or at the level of the wall defect. The sponge is connected to an external vacuum device through a nasogastric or nasocystic tube, which provides continuous negative pressure[6,18] This absorbs fluid, accelerates granulation tissue formation and early healing. The system is replaced every 2-5 days, when the defect is evaluated[6-8,14]. The clinical success rate ranges from 84% to 100%[14]. A combination of EVAC with SEMS has been described, which improves vacuum force and permits esophageal passage[14,16,24].
Figure 9. Leakage with endo-SPONGE treatment[11]. (A) Wall defect; (B) Endo-SPONGE placement.
If a septum between the perigastric fluid collection and the gastric lumen is identified, then septotomy is indicated[5,18]. Dividing this septum with an endoscopic needle knife, cutting knife or APC probe will facilitate drainage from the cavity into the stomach. This procedure is effective because exposing the leak cavity equalizes the pressures of the cavity and the stomach and prevents esophageal and gastric secretions preferentially going into the cavity[5,18]. Case reports show a 100% of success rate with this technique[18].
Bleeding
Bleeding can occur from the gastrointestinal tract (staple line or anastomosis), the port placement sites, dissection planes, splenic injury, omentum or mesentery transection areas. Incidence of bleeding is 1%-4% and in 85% of cases it resolves spontaneously[3-8,17]. This usually occurs during the first hours to days after surgery due to a technical error or patient-related factors including perioperative medications, including the need for full anticoagulation after surgery. Endoscopic therapy can be used to address intraluminal bleeding[6].
Tachycardia, hypotension, hemoglobin drop, hematemesis or hematochezia are common signs and symptoms of early postoperative bleeding[3-8]. In patients who are hemodynamically stable, standard supportive treatment should be initiated with resuscitation, possible blood products transfusion, serial hematocrit, correction of coagulopathies and stopping VTE prophylaxis if this is being used[3-5,17]. Endoscopy is considered when patients have proven bleeding refractory to supportive therapy. The sooner the bleeding (within the first 24 h) the higher the probability of hemodynamic instability requiring emergent surgical or endoscopic treatment[5,7,8]. Endoscopy has been proven to be safe in the early postoperative period[13].
Bleeding at the anastomosis or the staple line can be identified with the regular endoscope and treated simultaneously. Balloon or spiral assisted endoscopy or laparoscopy might be needed for distal bleeding at the JJ or gastric remnant[3-5]. It is likely that the most important factor in controlling early bleeding is mechanical compression[4,5]. Injection of saline, diluted epinephrine or various sclerosant agents and hemoclips (TTSC or OTSC) are the first options used[3-5]. These produce mechanical compression to help control bleeding. The volume of fluid injected tamponades the bleeding vessel. TTSC can also provide some compression by imbricating the surrounding tissue. OTSC or large volume liquid injections in most cases control the bleeding but might have a higher risk of a long-term stricture[3-5]. Thermal therapy (heater probe treatment, mono or bipolar electrocoagulation, and argon plasma coagulation) and application of hemostatic powder, fibrin or thrombin glue tend to be more successful in controlling diffuse bleeding[3-6]. Even though theoretically the risk of ischemia and necrosis with the use of sclerotherapy exists, it is extremely rare[3].
Stricture and stenosis
Stricture and stenosis occur between 3-4 weeks postoperatively[2,5,6,9]. The most common presenting signs and symptoms are dysphagia to solids that progresses to liquids, nausea, emesis, malnutrition, reflux, epigastric pain or significant weight loss over a short period. The incidence of strictures varies depending on the bariatric procedure performed, between 0.1%-3.9% for sleeves and 3%-28% for gastric bypass[2,5,6,14,25]. After a LSG, strictures occur at the incisura angularis or at the gastroesophageal junction, and after an RYGB, they usually occur at the GJ anastomosis (when less than 10mm in diameter) [2,5,6,25]. Early strictures can be due to surgical technique (linear vs circular vs hand sewn GJ anastomosis creation, improper placement of the staple line while creating the sleeve gastrectomy, partial or complete over-sewing of the staple line, torsion along the sleeve’s axis), surgical dehiscence, hematomas, ulceration, anastomotic tension, suture granuloma, or chemical agents (nonsteroidal anti-inflammatory drugs, NSAIDS, and tobacco)[2,6,25]. All of these factors increase fibrosis and retraction with the later development of a stricture. An upper endoscopy can definitively diagnose a stricture. A UGI series may be obtained to diagnose a stricture, but it only has a reported positive predictive value of approximately 66%. Endoscopy is more definitive and has the advantage of providing treatment at the time of diagnosis. Signs of stricture seen on endoscopy includes the presence of a stenotic lumen, dilation of the gastric pouch or proximal sleeve, and/or undigested food[2-6,25].
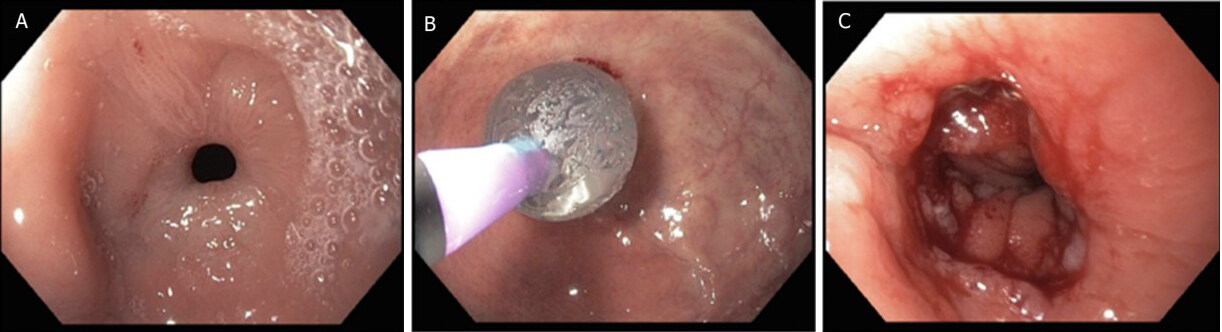
Endoscopic treatment consists of dilation of the stricture. Ideally, dilation should be done after 3-4 weeks postoperatively to avoid the risk of perforations[2-6,17]. Perforations occur in 2.2% of cases. Different options are available including through the scope balloon dilation [Figure 10] and bougienage, which can be done under fluoroscopy and over a guidewire when the stricture is too narrow. Dilations are performed in different sessions every 10-14 days. Most cases resolve after 2 or 3 sessions[2-6,24,25]. The balloon should be positioned at the site of maximal luminal narrowing, then it is expanded to its maximum diameter and held for one minute under tension. Bougienage consists of advancing progressively, increasing bougie sizes during the same endoscopic session. This technique, besides being reusable, gives the proceduralist the ability to assess the resistance of the stenosis and decide on whether a larger bougie can be advanced in a safe manner. GJ anastomosis should be dilated to 15-18 mm, no larger than that, to prevent weight regain[5,6]. Early strictures that are dilated within the first 3 months postoperatively have a higher probability of resolving with endoscopic treatment[23-27].
Figure 10. Balloon dilation of anastomotic stenosis[11]; (A) stenosis, (B) balloon dilation, (C) anastomosis after dilation.
Stents can also be used in the treatment of strictures. The stent success rate has been described to be 83%[23-27]. SEMS are most commonly used for both GJ anastomosis and sleeve strictures. They are placed over the stricture and left in place for 10-14 days to provide constant pressure and promote dilation. Exchange of the stent is done to prevent incorporation into the native tissue. The most common complication is stent migration. Recent case reports suggest that lumen-apposing metal stents (LAMS) are also successful in treating GJ anastomosis strictures. Its novel dumbbell design makes it less likely to migrate when compared to SEMS[23-27]. Further studies are needed to compare the efficacy of SEMS vs LAMS in the treatment of GJ anastomotic strictures.
Resistant strictures can be treated with stricturoplasty and/or steroid injections[23-27]. Strictures that persist after multiple endoscopic treatments require surgical revision. LSG needs to be converted to an RYGB and re-doing the GJ anastomosis is required for RYGB strictures[23-27].
Bezoar
Undigested fibers or milk products, coagulated blood, hair or medications found intraluminally are referred to as bezoar[2,5,6,11]. Bezoars that do not advance in the GI tract found after bariatric surgery may cause obstruction. In the early postoperative period, early clot, diet transgression or food not well-chewed can form bezoars, which might require surgical reintervention for an early bowel obstruction. After gastric bypass, if a bezoar is found in the GJ anastomosis, the patient can present with nausea, emesis, dysphagia, and excess salivation[5,11,12]. Some can tolerate liquids after episodes of emesis that give temporary relief. Endoscopy is used for diagnosis and treatment techniques like water jet fragmentation, direct suction, snare, net or baskets and drills[5]. Endoscopic graspers might be needed to aid in the retrieval of the bezoar transorally or to help it pass forward beyond the stricture site. Stricture at the GJ anastomosis or stenosis of the sleeve and foreign bodies at the staple lines serve as a nidus for bezoar formation[5].
SUMMARY
Metabolic and bariatric surgery is a safe and effective treatment for obesity and its comorbidities. Unfortunately, it is not complication-free. However, when they do occur, upper endoscopy is considered a safe, cost-effective and efficacious modality that can be used to diagnose and treat early postoperative complications. Proceduralist experience is an important factor in determining treatment alternatives and subsequent outcomes.
DECLARATIONS
Authors’ contributionsMade substantial contribution to the conception and design of the manuscript: Ardila-Gatas J, English W
Data was gathered, analyzed and interpreted based on authors experience on the field and evidenced based data
Availability of data and materialsData was obtained from a literature review of latest articles published on the subject. Types of articles included review articles, meta-analysis, cohort and case-control studies and case reports. All the articles were found on surgical journals as listed on the references.
Financial support and sponsorshipNone.
Conflicts of interestBoth authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med 2019;381:2440-50.
2. Cai JX, Schweitzer MA, Kumbhari V. Endoscopic management of bariatric surgery complications. Surg Laparosc Endosc Percutan Tech 2016;26:93-101.
3. Spota A, Cereatti F, Granieri S, et al. Endoscopic management of bariatric surgery complications according to a standardized algorithm. Obes Surg 2021;31:4327-37.
4. Lim R, Beekley A, Johnson DC, Davis KA. Early and late complications of bariatric operation. Trauma Surg Acute Care Open 2018;3:e000219.
5. da Rocha LC, Ayub Pérez OA, Arantes V. Endoscopic management of bariatric surgery complications: what the gastroenterologist should know. Rev Gastroenterol Mex 2016;81:35-47.
6. Ardila-gatas J, Pryor A. Endoscopic approach for the treatment of bariatric surgery complications. Mini-invasive Surg 2020;4:16.
7. Chang SH, Freeman NLB, Lee JA, et al. Early major complications after bariatric surgery in the USA, 2003-2014: a systematic review and meta-analysis. Obes Rev 2018;19:529-37.
8. Kassir R, Debs T, Blanc P, et al. Complications of bariatric surgery: presentation and emergency management. Int J Surg 2016;27:77-81.
9. Boules M, Chang J, Haskins IN, et al. Endoscopic management of post-bariatric surgery complications. World J Gastrointest Endosc 2016;8:591-9.
10. Kumar N, Thompson CC. Endoscopic management of complications after gastrointestinal weight loss surgery. Clin Gastroenterol Hepatol 2013;11:343-53.
11. Valli PV, Gubler C. Review article including treatment algorithm: endoscopic treatment of luminal complications after bariatric surgery. Clin Obes 2017;7:115-22.
12. MBSAQIP Data Registry. 2021 Qualified Clinical Data Registry. Available from: https://www.facs.org/ [Last accessed on 6 Apr 2022].
13. Sharma G, Ardila-Gatas J, Boules M, et al. Upper gastrointestinal endoscopy is safe and feasible in the early postoperative period after Roux-en-Y gastric bypass. Surgery 2016;160:885-91.
14. ASMBS-SAGES. Bariatric endoscopy skill acquisition focused evaluation (BE-SAFE) 2019. Available from: https://besafe.asmbs.org/ [Last accessed on 6 Apr 2022].
15. Fernandez-Esparrach G, Lautz DB, Thompson CC. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: a less-invasive approach. Surg Obes Relat Dis 2010;6:282-8.
16. Mansoor MS, Tejada J, Parsa NA, Yoon E, Hida S. Off label use of lumen-apposing metal stent for persistent gastro-jejunal anastomotic stricture. World J Gastrointest Endosc 2018;10:117-20.
17. Heneghan HM, Meron-Eldar S, Yenumula P, Rogula T, Brethauer SA, Schauer PR. Incidence and management of bleeding complications after gastric bypass surgery in the morbidly obese. Surg Obes Relat Dis 2012;8:729-35.
18. Gjeorgjievski M, Imam Z, Cappell MS, Jamil LH, Kahaleh M. A comprehensive review of endoscopic management of sleeve gastrectomy leaks. J Clin Gastroenterol 2021;55:551-76.
19. Hamed H, Said M, Elghadban H, Elgeidie A. Outcome and adverse events of endoscopic bariatric stents for management of leakage after bariatric surgery. Obes Surg 2020;30:982-91.
20. Giuliani A, Romano L, Marchese M, et al. Gastric leak after laparoscopic sleeve gastrectomy: management with endoscopic double pigtail drainage. A systematic review. Surg Obes Relat Dis 2019;15:1414-9.
21. Over the scope clips (OVESCO). Available from: https://ovesco.com/otsc-system/ [Last accessed on 6 Apr 2022].
22. Siddique I, Alazmi W, Al-Sabah SK. Endoscopic internal drainage by double pigtail stents in the management of laparoscopic sleeve gastrectomy leaks. Surg Obes Relat Dis 2020;16:831-8.
23. Chang SH, Popov VB, Thompson CC. Endoscopic balloon dilation for treatment of sleeve gastrectomy stenosis: a systematic review and meta-analysis. Gastrointest Endosc 2020;91:989-1002.e4.
24. Puli SR, Spofford IS, Thompson CC. Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc 2012;75:287-93.
25. Rosenthal RJ. Dilating the stenotic gastrojejunostomy after laparoscopic Roux-en-Y gastric bypass for morbid obesity: when things go wrong. J Gastrointest Surg 2009;13:1561-3.
26. Ramage JI Jr, Rumalla A, Baron TH, et al. A prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection therapy for recalcitrant esophageal peptic strictures. Am J Gastroenterol 2005;100:2419-25.
27. Moura DTH, Jirapinyo P, Aihara H, Thompson CC. Endoscopic tunneled stricturotomy in the treatment of stenosis after sleeve gastrectomy. VideoGIE 2019;4:68-71.
28. Boston Scientific esophageal stents and resolution clips. Available from: https://www.bostonscientific.com/en-US/products.html [Last accessed on 6 Apr 2022].
29. OverStitch endoscopic suturing system. Available from: https://www.overstitch.com/ [Last accessed on 6 Apr 2022].
30. Cook Medical Solus Double Pigtail Stent. Available from: https://www.cookmedical.com/products/esc_zss_webds/ [Last accessed on 6 Apr 2022].
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Ardila-Gatas J, English W. Endoscopic management of early complications following bariatric surgery. Mini-invasive Surg 2022;6:21. http://dx.doi.org/10.20517/2574-1225.2021.133
AMA Style
Ardila-Gatas J, English W. Endoscopic management of early complications following bariatric surgery. Mini-invasive Surgery. 2022; 6: 21. http://dx.doi.org/10.20517/2574-1225.2021.133
Chicago/Turabian Style
Ardila-Gatas, Jessica, Wayne English. 2022. "Endoscopic management of early complications following bariatric surgery" Mini-invasive Surgery. 6: 21. http://dx.doi.org/10.20517/2574-1225.2021.133
ACS Style
Ardila-Gatas, J.; English W. Endoscopic management of early complications following bariatric surgery. Mini-invasive. Surg. 2022, 6, 21. http://dx.doi.org/10.20517/2574-1225.2021.133
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 14 clicks
Cite This Article 14 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.