Management of gastrointestinal bleeding following bariatric surgery
Abstract
Gastrointestinal bleeding following bariatric surgery is a relatively rare adverse event but constitutes a significant cause of morbidity. It requires a high index of suspicion, early diagnosis, and prompt management, as it can lead to rapid deterioration and potential mortality. In most cases, GI bleeding is self-limited and does not necessitate emergent reoperation. For some patients, however, control of postoperative hemorrhage may require various procedural-based interventions via surgical, endoscopic, or radiologic approaches. Recent studies suggest that endoscopic therapies to manage intraluminal bleeding post-bariatric surgery are becoming increasingly popular given their high efficacy rate and favorable safety profile. Currently, there is no consensus on the management of early or late GI hemorrhage after metabolic surgery. Therefore, the aim of this review is to summarize the effectiveness of several treatment options and outline management algorithms for this subset of bariatric patients based on the established literature.
Keywords
INTRODUCTION
The number of adults diagnosed with obesity has been on the rise throughout the last few decades, reaching a prevalence in the US of 42.4% in 2017-2018[1]. This increase correlates with the rise of bariatric surgery as an effective and safe treatment modality for obesity and related comorbidities[2,3]. Despite the widespread adoption of minimally invasive techniques and institution of quality improvement metrics in bariatric surgery, postoperative complications still occur and are often associated with the underlying anatomic changes inherent to weight loss procedures. Classic examples include anastomotic leaks, fistulas, strictures, intestinal obstruction, thrombo-embolic events, and gastrointestinal bleeding[4,5].
Although a relatively rare adverse event, postoperative GI hemorrhage is a major cause of morbidity in the bariatric patient and should be addressed expeditiously. Significant delay in diagnosis or treatment could potentially result in rapid clinical deterioration and even mortality. Surgeons should be able to recognize the occurrence of this complication in a timely fashion, determine the clinical condition of the patient, appropriately evaluate the source of bleeding and formulate a customized treatment plan that considers the type of bariatric operation the patient has undergone and factors contributing to the development of postoperative GI bleeding. Although basic principles for the management of GI bleeding in the postoperative setting hold true, a more nuanced approach is needed as diagnosis, management, and treatment options for bariatric patients can vary significantly given the anatomic variability.
The most commonly performed bariatric operations in the US are sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB)[6-8]. The incidence of postoperative hemorrhage for these procedures is estimated to be 0.5%-5.8%[9-14]. For the majority of patients, GI bleeding post-bariatric surgery is self-limited and does not necessitate emergent reoperation. In some cases, however, control of postoperative hemorrhage may require surgical, radiologic, or endoscopic interventions. Endoscopic therapies to treat intraluminal bleeding post-bariatric surgery are becoming more frequently utilized as recent studies have deemed them a safe and effective option for reducing periprocedural morbidity[13,15,16]. Despite gaining significant traction, the endoscopic intervention has not yet become the standard of care in the management of intraluminal GI bleeding post-bariatric surgery, given theoretical concerns of anastomotic disruption and risk of perforation related to instrumentation and insufflation.
This review highlights the key aspects of appropriate identification, localization, and treatment of GI bleeding following RYGB and SG. A comprehensive analysis of the existing literature, along with the authors’ experience, is used to propose management algorithms for early and late postoperative bleeding following bariatric surgery.
INCIDENCE OF POSTOPERATIVE HEMORRHAGE
GI bleeding following weight loss surgery occurs in 0.5%-5.8% of cases[9-14]. Despite being a relatively infrequent complication, hemorrhage post-bariatric surgery is encountered by every practicing bariatric surgeon, and it remains the most common reason for reintervention within 30 days after the index operation[10]. Postoperative bleeding usually occurs in the setting of RYGB or SG, and is rarely seen after vertical banded gastroplasty and laparoscopic adjustable gastric banding[17-19]. In a recent meta-analysis involving 3464 RYGB patients, the postoperative hemorrhage rate was 1.9%[5], significantly lower than the 3.2% incidence previously reported by Bakhos et al.[20] in 2009. Including both laparoscopic and open approaches, the earlier study found minimally invasive techniques associated with a higher rate of hemorrhage (5.1% vs. 2.4%, P = 0.02)[20]. The higher incidence of GI hemorrhage after laparoscopic RYGB has been consistently reported in multiple studies[11-14]. Variability in postoperative hemorrhage rates can be impacted by several factors, including different surgical techniques and thresholds used to define and report a bleeding incident.
It is important to note the significantly high rate of rebleed in the setting of GI hemorrhage post-bariatric surgery. A retrospective study of 933 patients who underwent laparoscopic Roux-en-Y gastric bypass (LRYGB) found that 3.2% of patients experienced acute postoperative bleeding, with 63% of them subsequently developing recurrent bleeding episodes (2 episodes in 43%, 3 episodes in 10%)[16]. It is worth mentioning that, at the first bleeding episode, most patients were treated endoscopically (77%), 13% underwent Esophagogastroduodenoscopy (EGD) for diagnostic purposes, and 10% were treated supportively without receiving any endoscopy. During the second bleeding episode, 31% of the patients required EGD, from which 80% had already been treated endoscopically in the first episode. As such, therapeutic interventions are likely required for these patients. However, the overall mortality rate of bariatric surgery is very low (0.08%)[21], with continued improvements in the management of postoperative complications, including hemorrhage, contributing to its continuing decline[22,23].
Despite the clear benefit of bariatric surgery, endoscopic bariatric procedures performed using flexible endoscopy offer an alternative option for obesity treatment. This approach may be appropriate for patients who do not qualify for bariatric surgery (increased operative risks, cardiovascular complications, or BMI < 35 kg/m2 without comorbidities) or those who prefer a safer and less invasive treatment strategy. Therefore, these procedures have gained popularity, as shown by the increase from 2% to 4% of all bariatric procedures during the period 2014-2016[24]. Although endoscopic weight loss interventions carry a low risk of serious adverse events, they are still being reported in the literature. Post-interventional GI hemorrhage rates vary significantly based on the type of endoscopic procedure. Specifically, in the recent “REDUCE Pivotal Trial” investigating the outcomes and complications of Intragastric Balloon placement, GI bleeding was reported in two cases (1%). The source of bleeding was an ulcer at the gastroesophageal junction, treated with transfusion in the first patient, and an esophageal mucosal tear caused during balloon removal, which was managed with hemostatic clips[25]. Finally, based on recent data, the pooled GI hemorrhage incidence for endoscopic sleeve gastroplasty is 0.82%, with most patients being treated supportively with red blood cell transfusions[26].
Early vs. late postoperative bleeding
Generally, when GI hemorrhage develops within 30 days of surgery, it is characterized as early[27], while bleeding after the 30 days is considered delayed presentation. GI bleeding complications most frequently occur in the intraoperative or immediate postoperative period[28,29]. Some studies report that up to 70% of postoperative bleeding complications present within the first 4 hours after surgery[30]. Specifically, early bleeding after RYGB in 1%-5% of patients manifests within 48 hours[31], while in SG, this rate varies between 0% and 8%[32]. In contrast, postoperative bleeding rarely happens in LAGB, reaching a percentage of only 0.1%[32].
Because most postoperative hemorrhagic complications occur within the first few days, alternative bleeding classification criteria have been suggested. Specifically, Fridman et al.[33], in the American Society for Metabolic and Bariatric Surgery (ASMBS) textbook of bariatric surgery, proposed dividing bleeding into Acute (1-7 days), Early (1-6 weeks), Late (6-12 weeks), and Chronic (> 12 weeks) based on the time of presentation after the bariatric surgery. Nevertheless, the management of acute and early hemorrhage did not differ substantially, which was also the case for late and chronic postoperative bleeding; therefore, in this review, we use two categories of postop bleed, early and late.
The etiology of early and late GI bleeding differs substantially; thus, time of onset should be taken into consideration when developing treatment plans, as different diagnostic procedures and management options may be available. For SG, long-staple lines, short gastric vessel pedicles, and the trocars sites are the most common sources of bleeding in the early postoperative period[33]. In RYGB cases, bleeding usually originates from the staple line of the gastrojejunal (56%) or jejunojejunal anastomosis (11%), or the gastric pouch (33%)[15,34], however, other staple lines and trocar sites can also bleed. The mechanism for staple line bleeding is submucosal blood vessel damage which occurs from vessel transection at tissue edges or with vessel penetration during stapling[35]. In both SG and RYGB, incomplete hemostasis after surgical dissection may also cause hemorrhage.
In contrast, delayed bleeding usually occurs in the setting of marginal ulceration, causing intraluminal hemorrhage[36]. Marginal ulcers are classically located at the gastrojejunal anastomosis but can also occur less frequently in the gastric remnant or at the jejunojejunal anastomosis. The underlying pathophysiologic mechanisms leading to the development of anastomotic ulcers are poor tissue perfusion, loss of mucosal barrier, or increased acid production. This could be caused by surgical techniques such as increased tension during construction of the anastomosis, utilization of non-absorbable sutures, or factors that predispose to ulceration such as alcohol consumption, nicotine, non-steroidal anti-inflammatory (NSAIDs) or steroid medication, and enlarged gastric pouch or gastrogastric fistulae. The development of GI neoplasm is a rare cause of delayed GI bleeding but should also be considered.
MANAGEMENT OF EARLY POSTOPERATIVE BLEEDING
Diagnosis and supportive treatment
The initial evaluation of a patient with suspected clinically significant postoperative GI hemorrhage is to assess the severity of the bleed, detect the bleeding source, and recognize the presence of conditions that could affect management. In this context, physical examination should be a key aspect in assessing the extent of postoperative hemorrhage. Mild to moderate hypovolemia, defined as < 15% loss of blood volume, usually manifests as resting tachycardia, while blood loss > 15% or > 40% presents as orthostatic and supine hypotension, respectively[37]. Additionally, laboratory tests, including CBC, serum chemistries, liver tests, and coagulation, should be obtained. Patients should have nothing per mouth, and supplemental oxygen by nasal cannula should be administered. Adequate IV access should be established either with two large caliber (≥ 18 gauge) peripheral intravenous catheters or a central venous line. Adequate resuscitation and hemodynamic stabilization are essential prior to endoscopy to minimize treatment-associated complications[38].
Although endoscopy can be used for both diagnostic and therapeutic purposes, an abdominal imaging study may be needed to detect and differentiate active bleeding from hematoma or between intraluminal and extraluminal bleeding[20]. Specifically, in a recent study by Heneghan et al.[39], assessing bleeding complications of 4466 patients after RYGB, the most used diagnostic modality to detect the source of hemorrhage was endoscopy (47.6%), followed by imaging studies (CT scan, angiography) (35.7%), surgical exploration (33.3%), and clinical diagnosis (19%). 31% of patients with postoperative bleeding required multiple diagnostic modalities to locate the site of hemorrhage[39].
Patients experiencing clinically significant postoperative hemorrhage early after surgery generally present with signs of shock, including hypotension, tachycardia, and acute anemia. The key steps in management are prompt resuscitation, close monitoring of vital signs, cessation or reversal of anticoagulation, and consideration of blood transfusion. In most cases, acute postoperative hemorrhage is insignificant and resolves without surgical intervention[40]. In a case series of 22 patients with postoperative upper GI hemorrhage after LRYGB, 73% were managed supportively, while the remaining 27% required intervention[13]. Similarly, a larger retrospective study of 450 LRYGB patients reported that 85% of patients with postoperative hemorrhage did not require procedural intervention, with the remaining 15% undergoing reoperation[41]. Another study reviewing 89 LRYGB cases presenting with postoperative GI bleeding reported that 20% of patients had a self-limited hemorrhage, 55% required fluid replacement therapy and blood transfusion, and 20% underwent reoperation[28]. As such, establishing adequate venous access, fluid replacement with crystalloids, discontinuation of anticoagulants (resuming anticoagulation should be tailored to the patient’s risk for thrombosis and recurrent bleeding), and antiplatelet agents are critical first steps. This should be followed by hemodynamic monitoring, correction of any coagulopathies, administration of Proton Pump Inhibitors (PPI) (typically includes IV PPI every 12 hours or starting a continuous infusion), serial hematocrit measurements, consideration for blood product transfusions and observation for any additional bleeding episodes[6,42]. The role of prokinetic agents, such as erythromycin and metoclopramide, in acute upper GI bleeding has been studied and is thought to promote better visualization during endoscopy[43]. If a patient fails to respond to supportive treatment, has persistent bleeding, or clinical deterioration, then consideration of procedural interventions, including endoscopy, angiography, and reoperation, is warranted.
Endoscopic intervention
The use of therapeutic endoscopy early in the postoperative period had been debated for years due to concerns about iatrogenic damage to the anastomosis. However, over time it has started being implemented in clinical praxis more often to decrease postoperative complications[44]. In the context of GI bleed, EGD has become a popular diagnostic and therapeutic modality in managing early postoperative bleeding but remains a controversial area. Some experts argue against EGD early after surgery because of the risk of iatrogenic dehiscence and perforation at the gastrojejunal anastomosis[12,20,45]. Performing EGD in this setting is very challenging, and requires a high level of expertise and excellent knowledge of the post-surgical anatomy. Therefore, they prefer to proceed with diagnostic laparoscopy for exploration and treatment. In contrast, other clinicians suggest EGD as first-line therapy for post-bariatric surgery complications in patients that do not require urgent reoperation[46].
There has been increasing evidence that endoscopy is a very useful and safe tool to investigate and treat early intraluminal GI bleeding, as it allows direct visualization and evaluation of the pathology[15,39,47,48]. In a retrospective study, including 933 patients who underwent LRYGB, an EGD was performed in 27 patients due to postoperative Upper GI Hemorrhage (UGIH). In 24 cases (85%), endoscopic treatment (epinephrine injection, heater probe cautery, epinephrine injection plus thermal coagulation, and hemoclip) was applied, resulting in 100% hemorrhage control. However, a second EGD was required in 5 patients (17%) due to bleeding recurrence. In all cases, bleeding originated from gastrojejunal anastomosis. None of the 24 patients required surgery for hemorrhage control, but two endoscopic procedure-related complications occurred (a perforation and an aspiration), with one resulting in death and the second in reoperation for complication management[16]. In another review by Spaw and Husted, 11 of 89 patients (15.4%), who presented with postoperative bleeding after RYGB, required EGD for diagnosis (6 patients) and treatment (5 patients). Although no deaths occurred, a secondary EGD was necessary in one case to control bleeding at the jenuno-jejunal staple line[28]. Similar results were reported in a case series, where UGIH after LRYGB was managed endoscopically in 5 patients. Hemostasis was achieved in all cases by using epinephrine injections alone or epinephrine combined with polidocanol without any further complications[13].
However, endoscopic treatment has limited effectiveness when bleeding originates from the jejunojejunostomy or the excluded stomach since some patients have a long alimentary limb or the intraluminal blood clots obstruct visualization. Still, successful endoscopic management of bleeding at the jejunostomy has been described in the literature[47]. Because of these difficulties, interventions such as transgastric endoscopy or surgical exploration remain the basic diagnostic and therapeutic methods, as they are safe and effective[49,50].
Multiple hemostatic modalities are available for the endoscopic treatment of intraluminal bleeding in the early postoperative period[51]. Endoclips, also known as through-the-scope (TTS) clips, and epinephrine injections are the most frequently utilized by endoscopists, but they do not always achieve adequate hemostasis[52]. Endoclips apply mechanical pressure to tissues resulting in compression and bleeding cessation. Successful hemorrhage control with TTS clip application can be performed safely without any recurrent bleeding episodes or complications[48]. Endoclips are applied to bleeding vessels under direct visualization, can be easily removed if malpositioned, and additional clips may be placed in series along staple-lines or in the event of recurrent bleeding[48,53-55]. Hemoclips typically slough off in 2-4 weeks without causing tissue injury[53,56,57]. Although not primarily used for perforation management, proper hemoclip application with tissue approximation can also be used to treat small concomitant anastomotic leaks and iatrogenic perforations[48]. Successful closure of small gastric or jejunal perforations with the application of TTS clips has been described in the literature[58,59].
Endoclips are usually combined with other traditional hemostatic methods like injection therapy [Figure 1]. A case report published by Campos et al.[40] describes the effective treatment of significant intraluminal hemorrhage 12 hours post-RYGB with endoclips and diluted epinephrine (1:10,000) injections without complications or recurrence. The α-receptor-dependent vasoconstriction of epinephrine and the fibrosis caused by the local inflammatory response lead to platelet aggregation and hemostasis[60]. The increased injection volume causes mechanical tissue compression, resulting in tamponade and bleeding control. Therefore, using larger fluid volumes of dilute epinephrine solution has been associated with better hemorrhage control and recurrence rates[60]. It is important to note that the use of epinephrine injection alone has been proven to be less effective than combined hemostatic modalities for treating hemorrhagic episodes in the setting of peptic ulcer disease[61-63]. Similarly, in a recent network meta-analysis investigating dual-therapy for high-risk bleeding ulcers in non-surgical patients, the combination of epinephrine plus mechanical therapy had significantly lower rebleeding and reoperation rates than epinephrine plus sclerosant agents or epinephrine alone[64].
Figure 1. Early postoperative hemorrhage after primary RYGB. The bleeding episode occurred on the first postoperative day, and EGD was used for both diagnosis and treatment. The staple line at the gastrojejunostomy was detected as the source of bleeding, which was successfully controlled by epinephrine injections followed by endoclip placement; RYGB: Roux-en-Y gastric bypass; EGD: esophagogastroduodenoscopy.
Endoscopic thermal coagulation therapies consist of contact probes (heater and multipolar) and argon plasma coagulation. Larger diameter contact probes with slow coagulation are best for the treatment of bleeding ulcers[51], and when combined with epinephrine injections, show clinically significant hemostatic results[64]. While coaptive coagulation results in robust hemostasis[16], delayed full-thickness injury to the staple-line or thin-walled jejunum can result in leak or perforation. Nonthermal techniques such as clip application and injection therapy are generally preferred in this scenario[55].
As previously described, the majority of intraluminal bleeds amenable to endoscopic management are successfully treated with some combination of endoclip application, injection therapy, and thermal coagulation. If adequate hemostasis cannot be achieved with these methods, however, the advanced endoscopist may still have several potential treatment options available. These innovative therapies include the application of over-the-scope (OTS) clips, hemostatic sprays, and flexible endoscopic suturing.
The clinical application of OTS clips was initially described in a case series by Kirschniak et al.[65] to treat refractory bleeding, iatrogenic perforations, and other deep wall lesions. In all cases, the treatment was successful, resulting in hemostasis and effective lesion closure, while no adverse events were reported[65]. The advantage of OTS clips is that because of their bigger size (up to 14 mm), they can control the bleeding of larger and more complex lesions with the deployment of a single clip[66,67]. At the same time, they produce greater tensile strength leading to effective hemostasis[68]. Although OTS clips are being used for a wide variety of conditions, they have been successful in the management of upper GI hemorrhage[68,69]. In a recent retrospective study of 100 patients presenting with non-variceal UGIH, the primary hemostasis rate was 94%, and it reached 86% during the follow-up[70]. Similarly, the primary hemostasis rate reported by
Another alternative treatment modality for controlling postoperative hemorrhage is the application of hemostatic powder (hemospray). It was recently approved for use in the endoscopic treatment of GI bleeding since it is effective, especially in cases with a large bleeding surface where conventional endoscopic modalities fail to control hemorrhage. This is a novel treatment thought to cause hemostasis by sealing injured blood vessels and activating platelets and the intrinsic coagulation pathway[73]. It is being applied for the treatment of upper or lower GI bleeding. When used as monotherapy, the hemostasis rate is high, but bleeding tends to recur. In a cohort study, immediate bleeding control, although not in the postoperative setting, was achieved in 88.4% of patients with upper or lower GI bleeding; however, 33.7% experienced rebleeding[74]. This tendency was confirmed by a recent meta-nalysis including 1916 patients. The hemostasis rate after hemospray application was 94.5%, but the recurrence percentage within 30 days after therapy was still high (17.6%)[75].
Finally, newer strategies to control GI bleeding post-bariatric surgery, such as flexible endoscopic suturing, have been proposed, especially when traditional endoscopic measures, like endoclips, fail to cause hemostasis. The OverStitch Endoscopic Suturing System allows physicians to place full-thickness sutures endoscopically and provide a secure approximation of tissue in contrast to endoclips, which approximate just the mucosa and thus are not useful in larger defects. Therefore, OverStitch can be used to treat a wide range of pathologies, including but not limited to gastrointestinal tract perforations, fistulas, and anastomotic leaks[76-82]. Successful application of through-the-scope stitching technique has been reported in the past to treat perforated duodenal ulcers, leaks, and upper GI bleeding without significant complications[83]. In a recent case series, ten patients underwent endoscopic suturing to control peptic ulcer-related upper GI hemorrhage. In nine patients, previous endoscopic treatment did not result in hemostasis. Nevertheless, the application of endoscopic suturing resulted in bleeding control in all cases, with no early or late rebleeding and no adverse events[84]. Further, in a case report, massive hemorrhage due to marginal ulcer 10 days after RYGB was managed successfully with endoscopic sutures without reporting any rebleeding episodes or complications[85]. The outcome data on the use of the Overstitch device for hemostasis in the GI tract are limited, and the use of overstitch devices is restricted to the cases of previous failed endoscopic treatments or when the patient is at high risk for reoperation. However, the currently available data are very encouraging for the future application of endoscopic suturing systems in the early and late management of postoperative bleeding.
The role of tranexamic acid therapy
Tranexamic acid (TXA) is a potent antifibrinolytic agent that binds plasminogen, inhibiting plasmin formation and displacing plasminogen from the fibrin surface[86]. Activation of this pathway ultimately results in inhibition of fibrinolysis and blood clot formation. In the orthopedic literature, TXA has been shown to effectively reduce the overall number of hemorrhagic adverse events by decreasing bleeding and transfusion rates[87-89]. It has also been shown to lower the mortality rate in bleeding trauma patients undergoing surgery if given within the appropriate therapeutic treatment window[90]. In bariatric surgery, prophylactic TXA has been given in an attempt to reduce staple line bleeding[90]. In a study including laparoscopic sleeve gastrectomy (LSG) patients, the group treated with prophylactic tranexamic acid intraoperatively required significantly fewer stitches to stop the bleeding, had lower blood loss and shorter operation duration than the control group. However, no difference in the morbidity and mortality of the patients was detected[91]. Similar experiences were described by Hussain and colleagues[92], who used TXA routinely to reduce intraoperative bleeding in gastric bypasses, sleeve gastrectomies, and all revisional surgeries. Their experience after treating over 750 patients was that TXA administration during anesthesia induction and 8 hours after surgery had reduced the transfusion rate close to zero (1% without TXA)[92].
Therapeutic TXA administration for the treatment of bariatric patients with postoperative hemorrhage was studied retrospectively by Klaassen et al.[93] A total of 44 patients with bleeding after LRYGB or LSG received TXA. 4 patients (9%) ultimately required reoperation to achieve hemostasis, and 19 (42.2%) received a blood transfusion due to significant decrease in hematocrit[93]. No complications were reported within the 6-month follow-up, including any thromboembolic event or acute kidney injury. All patients received Low Molecular Weight Heparin (LMWH) prophylactically after surgery. However, the LMWH administration was not stopped in all patients after the hemorrhagic adverse event. In a subgroup analysis, patients that continued LMWH compared to those that discontinued LMWH had a greater reoperation rate, which, in part, may reflect the relatively high need for revisional surgery in this series. The authors concluded that TXA administration in the post-bariatric surgery hemorrhage setting might be safe and effective to reach hemostasis.
While no RCTs have yet been conducted to investigate the application of TXA in post-bariatric GI hemorrhage, several RCTs do exist for upper GI bleeding[94-103]. A review by Gluud et al.[104], using data from seven RCTs, was able to show the association of TXA with a decrease in mortality compared to the placebo-treated group. The more recent HALT-IT randomized, double-blinded, placebo-controlled trial investigated the effects of TXA on the treatment of upper GI bleeding in > 12,000 patients. TXA not only failed to demonstrate a significant difference in the mortality rate compared to placebo, but was also associated with significantly higher venous thromboembolic events (0.8% TA vs. 0.4% Placebo; RR 1.85; 95%CI: 1.15-2.98)[94]. In the same context, a meta-analysis of the 12 existing RCTs found no significant difference in mortality rate, rebleeding, need for surgery, and transfusion rate, while the risk of venous thromboembolic events with TXA was increased (RR 1.94; 95%CI: 1.23-3.05)[105].
Given the increased risk of thromboembolic events and its uncertain effectiveness in treating postoperative GI bleeding, TXA use remains controversial and is not routinely recommended for hemorrhage following bariatric surgery. The relatively small sample sizes in the studies, as mentioned earlier, may limit the ability to detect rare complications, such as thromboembolic events. Additionally, the lack of randomized controlled trials makes it difficult to come to certain conclusions regarding the efficiency of TXA in the post-bariatric bleeding setting as an alternative to reoperation.
Proposed treatment algorithm of early postoperative hemorrhage
Taking the prior described literature into consideration, most of the cases with early postoperative GI bleeding are self-limited, requiring only supportive treatment and close monitoring. In hemodynamically unstable patients, emergent surgical exploration is indicated, while in clinically stable patients, the source of bleeding should be investigated further prior to intervention. As previously mentioned, the most likely etiology of hemorrhage can often be deduced based on the timing of presentation. Similarly, patients’ symptoms and type of operation can help surgeons identify the likely site of GI bleeding. The first step is to identify if the bleeding is intraluminal, contained within the GI tract, or extraluminal [Figure 2].
Figure 2. Proposed algorithm for the management of early hemorrhage after bariatric surgery. *defined as persistent systolic BP < 100 mmHg, cardiac index < 2.2 L/min/m2, SvO2 < 60%, suspected pericardial effusion with tamponade physiology, base deficit > 8 mEq/L, or lactate > 5 mg/dL despite persistent inotropic, vasopressor, and/or volume resuscitation, and concern for or known right ventricular failure.
Extraluminal hemorrhage accounts for more than half of the postoperative bleeding instances and should be suspected in the absence of upper or lower GI bleeding symptoms but continuing clinical and laboratory signs of blood loss[20]. Diffuse abdominal tenderness with hypoactive bowel sounds, abdominal distension, tachycardia, rapid fall of hematocrit, and oliguria should prompt consideration of extraluminal blood loss[20]. While no longer routinely placed, the presence of a surgical drain with bloody output would also be indicative of the diagnosis. If present, the rate of bleeding should be determined, and the need for blood transfusion should be assessed. Specifically, in patients with active bleeding or hypovolemia, blood transfusion should be guided based on hemodynamic stability, estimated blood loss, rate of bleeding, and ability to stop the hemorrhage. However, for most stable patients with no active bleeding, transfusion is indicated if the hemoglobin levels are < 7 g/dL[106]. Patients with comorbidities (e.g., coronary artery disease) or higher risk for adverse events should have a goal hemoglobin of ≥ 8 g/dL.
It is important to note that the absence of bloody output does not exclude extraluminal hemorrhage, as this could result from a clogged or malpositioned drain[30]. Since extraluminal hemorrhage can present without any early and obvious symptoms after bariatric surgery, a high level of suspicion is necessary. When suspected, prophylactic anticoagulants should be discontinued, blood transfusion considered, and an abdominal CT obtained in a timely fashion[107,108]. If the extraluminal hemorrhage is confirmed or the patient does not improve with supportive treatment, the need for surgical reintervention should be considered. Although reoperation is the standard of care in the setting of clinical instability, CT-guided percutaneous procedures (e.g., hematoma evacuation, drainage of fluid collections) could prevent emergent exploration in stable patients[109]. In select cases, angiography could be considered as an alternative option for diagnosing and treating extraluminal bleeding but is infrequently employed and carries a high risk of ischemic anastomotic complications[44].
Although intraluminal bleeding post-bariatric surgery occurs less often than extraluminal bleeding[30], classic findings such as hematemesis, hematochezia, and melena, make its detection more straightforward[39]. Specifically, in RYGB patients, bleeding at the gastrojejunostomy or gastric pouch will most likely present with hematemesis[110]. Hematochezia or melena may indicate bleeding from the gastric remnant or jejunojejunal anastomosis[110]. Small bowel obstruction caused by intraluminal blood clots has also been described as a presenting symptom in patients with intraluminal hemorrhage after bariatric RYGB[111-113].
In SG patients, the localization of extraluminal bleeding is simpler as it generally originates from the staple line or the divided short gastric vessels[114]. Occasionally, the mucosa of the oropharynx, esophagus, or the lesser curvature of the stomach may be damaged during the application of bougie and result in early postoperative intraluminal bleeding. In this case, blood clots may need to be removed endoscopically to avoid obstruction[114]. Although supportive management of GI bleeding after SG is the key to controlling hemorrhage, staple line reinforcement has been suggested to reduce bleeding. In this context, a recent meta-analysis demonstrated that staple line reinforcement with buttressing was associated with less intraluminal hemorrhage after LSG and lower overall blood loss[115]. Further, a retrospective analysis of 522 patients undergoing LSG showed that the hemorrhagic complication rate was decreased when oversewing of the staple line with running suture was performed (no reinforcement vs. oversewing of staple line; OR: 3.34; 95%CI: 1.21-9.21)[116].
In a recent study, Sabry et al.[117] investigated the hemorrhagic complications after SG. The researchers reviewed 1500 patients identifying overall 80 cases with postoperative blood loss. The vast majority were extraluminal (95%), while only 5% occurred intraluminally. Initially, all patients were treated supportively with fluid resuscitation, monitored closely with lab tests, and an ultrasound of the abdomen and pelvis was performed in certain cases. These interventions were adequate to manage all intraluminal hemorrhage cases. In contrast, in 36 patients (45%) with intra-abdominal hemorrhage, urgent exploratory laparoscopy was performed to detect the bleeding site. In the extraluminal group, the most common bleeding source was the suture line (71%), followed by the omentum (11%), trocar insertion sites (8%), short gastric blood vessels (5%), and pancreatic adhesions (5%). In all patients with bleeding from the suture line, hemostasis was achieved with sutures and clips. For the other bleeding sites, cauterization, clips, and ligation of the bleeding points were successfully performed. All cases were identified and treated early, as they presented within the first 6 hours from surgery. This early management resulted in decreased infection rates, length of stay, need for blood transfusion, and faster patient recovery.
As mentioned in the previous study, after the intraluminal hemorrhage has been confirmed, the next step is to determine the severity of bleeding based on clinical signs, labs, and imaging studies. In the setting of clinically significant intraluminal hemorrhage, persistent bleeding that does not resolve with supportive treatment, or patients with episodes of recurrent bleeding, endoscopy (EGD) should be performed by a highly skilled interventionalist for diagnostic and therapeutic purposes. Always minimal insufflation, ideally with CO2, should be used to avoid disruption[31,44]. If the bleeding source is of gastrojejunal anastomosis origin, endoscopic therapy should be attempted. Numerous treatment modalities are available, including TTS or OTS clips, injection of diluted epinephrine or sclerosing agents, thermal coagulation, and application of hemostatic powder, fibrin, or thrombin glues[6,27,36,52,118]. If endoscopic treatment fails or recurrent bleeding occurs, repeat endoscopy or angiographic intervention can be considered. If at any point, the patient becomes clinically unstable or if EGD cannot be used (e.g., massive intraluminal clot), surgical intervention is required.
During endoscopic treatment of intraluminal hemorrhage, it is vital to recognize that post-bariatric patients are at higher risk for aspiration during the procedure. The combination of hematemesis, which in some studies has been reported as the most common presenting symptom (73%)[16], and the small gastric pouch in RYGB cases contribute to this complication. In fact, aspiration during therapeutic EGD under sedation has resulted in the death of patients with UGIH in the past[16]. Therefore, several bariatric surgeons support conducting EGD in the operating room under general anesthesia to avoid any delays in the case of emergency surgical exploration[16,40]. Additionally, endotracheal intubation should protect the airway and decrease the risk of aspiration.
Gastrojejunal anastomosis is the most common site of bleeding after RYGB[31]. However, if the EGD fails to locate the bleeding source or the patient has continuing symptoms of hemorrhage, other bleeding sites, such as the jejunojejunal anastomosis or the gastric remnant, should be considered. In these cases, the application of endoscopic therapy is limited, and therefore, surgical exploration with subsequent hematoma evacuation may be preferable. Nonetheless, laparoscopically assisted endoscopy after the RYBG procedure has been described as a possible diagnostic and therapeutic option[49,119]. If all the above measures fail to control hemorrhage, then resection of the gastric remnant could be required for definitive treatment.
The authors’ approach
The authors’ approach to early bleeding after bariatric surgery, as also outlined in the proposed algorithm, starts with the determination of the patient’s clinical condition. If the patient’s hemodynamic status has been minimally impacted by the bleeding, conservative management is employed with fluid resuscitation and blood transfusion as necessary. However, in the face of hemodynamic instability or ongoing bleeding despite conservative measures, the authors prefer to bring the patient back to the operating room, secure the airway via intubation, and perform an upper endoscopy for likely intraluminal bleeding or laparoscopic exploration for likely extraluminal bleeding (a CT scan should have been obtained if the patient was not unstable), or both if the source is unclear. For intraluminal bleeding, the clot is aspirated endoscopically, and the source of bleeding is sought and identified. Endoscopic clipping is used to control the bleed, and if unsuccessful, laparoscopy is performed and intracorporeal sutures are placed as needed until hemostasis is obtained. If the source of bleeding is not identified in the pouch or gastrojejunal (GJ) anastomosis and no bleeding is observed from the staple lines, the remnant staple line will be oversewn, and the jejunojejunostomy will be explored by opening the blind end of the biliopancreatic limb and inspecting the anastomosis from the inside. For extraluminal bleeding, the source will be identified after the clot has been suctioned out and addressed either with sutures or clips. Hemostatic agents may also be applied. In our own experience, this approach has always identified the source of bleeding, and we have been able to address it effectively without having to return to the operating room for rebleeding. It should also be noted that the use of an overtube may be very useful in these cases as multiple endoscope insertions may be needed to evacuate the clot. Finally, it is important that bariatric surgeons themselves are comfortable and have experience with endoscopic treatments of GI bleeding as it is vital for the treatment of these patients. Available structured courses, such as the BE-SAFE of the ASMBS[120], provide a great introduction to bleeding stopping techniques that every bariatric surgeon should be experienced in.
The authors would also like to highlight the importance of bleeding prevention. As necessary, approaches such as staple line oversewing or surgical clip application should be used liberally to perfect staple line hemostasis before leaving the operating room. Although clinical studies present controversial findings on the benefit of oversewing the staple line in RYGB[121], recent studies suggest that this method decreases both the incidence and the severity of postoperative hemorrhage[122]. Further, reinspection of the staple line several minutes after its initial creation will often reveal bleeding areas that are not immediately obvious. Staple line reinforcement or application of hemostatic agents are alternate approaches that, in the authors’ experience, are not as reliable as clip application and oversewing, however. Finally, the construction technique of the gastrojejunostomy is one of the most important steps in preventing postop bleeding, as stapled techniques are generally associated with a higher incidence of postop bleeding compared to hand-sewn techniques[123,124].
MANAGEMENT OF LATE POSTOPERATIVE BLEEDING
Late hemorrhage after bariatric surgery is a rare condition that has been reported in the literature, most frequently after RYGB[125-129]. The most common underlying pathology is the presence of marginal ulcers at the gastrojejunostomy [Figure 3], while gastritis, ulcers in the excluded stomach, duodenum, and pouch constitute other less common causes[130]. The incidence of marginal ulcers is higher in One Anastomosis Gastric Bypass (OAGB) compared to RYGB, and this should be taken into consideration when treating late postoperative GI hemorrhage[131]. The vast majority of late hemorrhagic complications, occurring after 30-days postoperatively, are intraluminal and usually present within the first several months[132-135]. Some studies suggest that the rate of marginal ulceration is higher in the laparoscopic compared to the open RYGB (4.1% vs. 12.3%)[136]. Late postoperative hemorrhage can present with a wide variety of signs and symptoms, including acute upper or lower GI bleeding, epigastric or abdominal pain, nausea, vomiting, iron deficiency anemia, and heme-positive stools[27].
Figure 3. Late postoperative hemorrhage after primary RYGB. The patient presented with hematemesis, epigastric abdominal pain, nausea/ vomiting, and anemia 4 years after a primary RYGB procedure. EGD was performed, and ulceration at GJA was detected with recent but not active bleeding signs. The ulcer bed was covered by fibrin, which was left in place. Definitive treatment was achieved with GJA revision due to recurrent bleeding. RYGB: Roux-en-Y gastric bypass; EGD: esophagogastroduodenoscopy; GJA: gastrojejunal anastomosis.
The first step in managing those patients is supportive care, similar to early postoperative bleeding control, and close monitoring of the vital signs and the lab values. Because ulcers remain to date the most common cause of late postoperative hemorrhage, identifying and modifying risk factors for ulcer development, especially smoking and chronic NSAIDs administration, is essential for successful treatment and positive response to medical therapy. Specifically, Azagury et al.[137] reported that 68% of patients treated with PPI plus risk factor modification for marginal ulcers after RYGB responded adequately to treatment. If the diagnosis is uncertain, the patient is unstable or fails to respond to medical therapy; then endoscopy is the intervention of choice [Figure 3]. The advantage of endoscopy is that it allows the assessment of ulcers in terms of size, depth, penetration, or presence of remaining sutures or staples within the lesion. Moreover, as in early hemorrhagic management, EGD gives the ability to control the bleeding using injection, thermal, and mechanical therapeutic modalities[45]. If the location of the bleeding ulcer is the gastric pouch or the gastrojejunal anastomosis, endoscopic treatment combined with intravenous PPIs is indicated.
In RYGB patients with a negative EGD for bleeding, the gastric remnant, the duodenum, or the jejunojejunostomy should be suspected as possible locations of GI hemorrhage, especially when it is combined with melena[44]. In these cases, imaging with a CT scan of the abdomen may provide information about the location of the bleeding by revealing the presence of an intraluminal clot and its location and/or signs of inflammation related to the presence of an ulcer. A bleeding scan could be an alternative diagnostic method if all previous workups fail to localize the bleeding site. Specifically, a Technetium-99m (99mTc) red blood cell scan was successfully used in a case of late GI hemorrhage following RYGB. Although the bleeding scan failed to show the exact location of the hemorrhage, it demonstrated a possible focus. This led the surgeons to perform a laparoscopic assisted endoscopy of the gastric remnant and identify multiple ulcers as the cause of bleeding[138].
Further, double-balloon enteroscopy may have a role, as described in a case report by Puri et al.[139], where a patient presented 6 months after RYGB with recurrent episodes of GI bleeding associated with melena, fatigue, dizziness, and shortness of breath. After negative upper endoscopy and colonoscopy, the patient underwent double-balloon enteroscopy. A bleeding duodenal ulcer and a vascular ectasia in the gastric remnant were identified and managed with bipolar electrocoagulation probe and clips. Hemostasis was not achieved in the excluded stomach, and finally, a gastrectomy was performed without any complications and bleeding recurrence[139]. As illustrated by this case, duodenal ulcers must always be included in the differential diagnosis of GI bleeding and cannot be evaluated by upper endoscopy in the RYGB patient. In this setting, double-balloon enteroscopy allows for visualization of the duodenum and the excluded stomach and is a viable alternative to intraoperative transgastric endoscopy or reoperation, which confer additional risk to the patient[140-143]. It is a rather infrequently performed and technically challenging procedure due to the anatomical difficulties in OAGB or RYGB procedures. However, it should be performed by an advanced endoscopist with experience in bariatric procedures.
For patients with persistent ulceration, recurrent bleeding, hemodynamic instability, or those who failed to be treated endoscopically, surgical intervention is necessary, requiring a gastrojejunostomy revision. The gastric pouch is transected proximal to the disease area and a new gastrojejunostomy is constructed. Other treatments, such as angiography and arterial embolization, have been described in the literature but should generally be avoided because of the risk of causing ischemia to the gastric pouch during the embolization of the left gastric artery. However, this intervention could be considered in high-risk patients for surgery[126]. In patients with persistent ulcer bleeding from the gastric remnant, resection may be required.
In a case series, four patients presented with severe late GI hemorrhage several years after RYGB (mean of 15.5 years). The reported symptoms were melena with or without associated syncopal episodes. Several diagnostic modalities (EGD, mesenteric angiography and nuclear scintigraphy) failed to localize the source of bleeding, and therefore, abdominal exploration with intraoperative endoscopy was performed. In all cases, bleeding originated from the excluded stomach, and total or subtotal gastrectomy was conducted with the resolution of symptoms and no further complications[126].
Although reports of late hemorrhage after RYGB have been inconsistent in the literature, some retrospective studies still exist. Specifically, Rabl et al.[125] followed up on 742 patients after RYGB and reported 7 (0.9%) cases with late hemorrhagic complications and a mean time from surgery to the occurrence of 62.6 months. 85% of patients had typical presenting symptoms of hematemesis or melena, caused by ulcers in 71%. EGD was the diagnostic intervention of choice for all patients, with a subgroup undergoing additional tests such as CT scan (43%) and laparoscopic transgastric endoscopy (29%). The bleeding location was the gastrojejunostomy in 57% and it was controlled with endoscopic epinephrine injections in two cases (29%), with transgastric endoscopy and argon coagulation in one (14%), while reoperation was required in four patients (57%). Similarly, Heneghan et al.[39], investigating bleeding complications after RYGB in 4466 patients, reported 12 late hemorrhages occurring after 30 days. The majority were intraluminal (83%), 66% of the cases were due to marginal ulceration, and no staple line bleeding was detected. Although EGD was used for diagnosis in over half of the patients, no endoscopic treatment was attempted. Instead, reoperation was performed in 33% of the patients, while the rest received only supportive treatment, including blood transfusion[39].
Finally, Gupta et al.[130], in a report on two cases with late hemorrhage after RYGB, presents some complications of advanced marginal ulcers. In both cases, the ulcer was detected at the gastrojejunal anastomosis, which had, as a consequence, the formation of a gastrogastric fistula at posterior wall of the excluded stomach. This led to perforation, erosion of the pancreas and splenic artery, and subsequent rapid patient deterioration. The initial diagnosis was made endoscopically, and treatment with epinephrine injections and/or endoclips was attempted in addition to supportive measures. Eventually, both required an exploratory laparotomy because of hemodynamic instability. Partial gastrectomy and a GJ reconstruction were performed, resulting in symptom resolution[130]. These cases show that the presentation of late hemorrhage after bariatric surgery can be severe and very challenging to manage. Therefore, early detection and emergent management are crucial. Although supportive care, endoscopic interventions as described previously, or angiography may be appropriate first steps, treatment should be tailored according to the severity and bleeding site of each patient.
The authors’ approach
The authors approach late bleeding by first addressing its underlying etiology, i.e., treating the anastomotic ulcer that caused the bleeding using iv PPI treatment in hemodynamically stable patients. An upper endoscopy is pursued if bleeding continues despite initial treatment or in patients with hemodynamic instability where the aforementioned endoscopic hemostatic approaches are employed. Operative intervention is rarely necessary and only needed if endoscopic approaches fail.
Further, the authors would like to emphasize the importance of bleeding prevention. Given that most of such bleeding originates from marginal ulcers after gastric bypass, PPI use to prevent them may be paramount. In retrospective studies, prophylactic PPI administration after RYGB surgery has been shown to decrease the incidence of marginal ulceration compared to control (1.2% vs. 7.3%; P = 0.001) and gastrointestinal complaints postoperatively[144]. These findings align with a recent meta-analysis, which demonstrated a lower rate of marginal ulcers after RYGB in patients taking PPIs vs. no PPI (OR: 0.50; 95%CI: 0.28-0.90; P = 0.02)[145]. Further, prophylactic PPI administration for 3 months vs. 1 month has been shown to be superior in preventing symptomatic marginal ulcers[146]. Although the latest guidelines of the ASMBS suggest considering prophylactic PPI after RYGB, further studies are necessary to define optimal practices for PPI prophylaxis (e.g., the optimal duration for PPI administration, agents, dosage, etc.) and maximize the clinical benefit for patients.
CONCLUSION
The incidence of postoperative hemorrhage following bariatric surgery continues to decrease. Effective management of this complication requires a high index of suspicion, expedient diagnosis, and an appropriate selection of available treatment options. This review analyzes the currently available surgical and non-surgical methods to control post-bariatric surgery gastrointestinal bleeding based on the presenting symptoms, postoperative anatomy, and the time of presentation. In this context, EGD constitutes an effective diagnostic and treatment tool for both early and late post-bariatric hemorrhage and should be attempted as soon as the patient has been stabilized. Except for the traditional hemorrhage control options (e.g., endoscopic epinephrine injections, endoclips), additional treatment methods (e.g., OTS, hemostatic powder) could be useful. Finally, pragmatic treatment algorithms are proposed for the management of early and late hemorrhage after bariatric surgery to maximize treatment success and, at the same time, minimize rebleeding episodes, possible complications, and the need for reoperation.
DECLARATIONS
Authors’ contributionsThe authors confirm contribution to the paper as follows: study conception and design: Giannopoulos S, Pokala B, Stefanidis D
Data collection: Giannopoulos S, Pokala B
Analysis and interpretation of results: Giannopoulos S, Pokala B, Stefanidis D
Draft preparation: Giannopoulos S, Pokala B
Critical content revision: Pokala B, Stefanidis D
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable
Copyright© The Author(s) 2022.
REFERENCES
1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief 2020;360:1-8.
2. Flum DR, Belle SH, King WC, et al. Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361:445-54.
3. Sjöström CD, Lissner L, Wedel H, Sjöström L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS intervention study. Obes Res 1999;7:477-84.
4. Murr M, Rafiei A, Ajami H, Fakhry TK. Overview of emerging concepts in metabolic surgery. Perm J 2010;14:57-62.
5. Podnos YD, Jimenez JC, Wilson SE, Stevens CM, Nguyen NT. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg 2003;138:957-61.
6. Cai JX, Schweitzer MA, Kumbhari V. Endoscopic management of bariatric surgery complications. Surg Laparosc Endosc Percutan Tech 2016;26:93-101.
7. ASMBS. Estimate of bariatric surgery numbers, 2011-2019. Available from: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers [Last accessed on 12 Apr 2022].
8. Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery survey 2018: similarities and disparities among the 5 IFSO chapters. Obes Surg 2021;31:1937-48.
9. Kitahama S, Smith MD, Rosencrantz DR, Patterson EJ. Is bariatric surgery safe in patients who refuse blood transfusion? Surg Obes Relat Dis 2013;9:390-4.
10. Augustin T, Aminian A, Romero-Talamás H, et al. Reoperative surgery for management of early complications after gastric bypass. Obes Surg 2016;26:345-9.
11. Papasavas PK, Hayetian FD, Caushaj PF, et al. Outcome analysis of laparoscopic Roux-En-Y gastric bypass for morbid obesity. The first 116 cases. Surg Endosc 2002;16:1653-7.
12. Nguyen NT, Rivers R, Wolfe BM. Early gastrointestinal hemorrhage after laparoscopic gastric bypass. Obes Surg 2003;13:62-5.
13. Fernández-Esparrach G, Bordas JM, Pellisé M, et al. Endoscopic management of early GI hemorrhage after laparoscopic gastric bypass. Gastrointest Endosc 2008;67:552-5.
14. Oliak D, Ballantyne GH, Weber P, et al. Laparoscopic Roux-en-Y gastric bypass: defining the learning curve. Surg Endosc 2003;17:405-8.
15. Sharma G, Ardila-Gatas J, Boules M, et al. Upper gastrointestinal endoscopy is safe and feasible in the early postoperative period after Roux-en-Y gastric bypass. Surgery 2016;160:885-91.
16. Jamil LH, Krause KR, Chengelis DL, et al. Endoscopic management of early upper gastrointestinal hemorrhage following laparoscopic Roux-en-Y gastric bypass. Am J Gastroenterol 2008;103:86-91.
17. Papakonstantinou A, Terzis L, Stratopoulos C, et al. Bleeding from the upper gastrointestinal tract after Mason’s vertical banded gastroplasty. Obes Surg 2000;10:582-4.
18. Pérez EM, Larrañaga E, Serrano P. Bleeding gastric pouch ulcer after vertical banded gastroplasty: a rare complication. Obes Surg 1997;7:454.
19. Iqbal M, Manjunath S, Seenath M, Khan A. Massive upper gastrointestinal hemorrhage: an unusual presentation after laparoscopic adjustable gastric banding due to erosion into the celiac axis. Obes Surg 2008;18:759-60.
20. Bakhos C, Alkhoury F, Kyriakides T, Reinhold R, Nadzam G. Early postoperative hemorrhage after open and laparoscopic Roux-En-Y gastric bypass. Obes Surg 2009;19:153-7.
21. Alam M, Bhanderi S, Matthews JH, et al. Mortality related to primary bariatric surgery in England. BJS Open 2017;1:122-7.
22. Smith MD, Patterson E, Wahed AS, et al. Thirty-day mortality after bariatric surgery: independently adjudicated causes of death in the longitudinal assessment of bariatric surgery. Obes Surg 2011;21:1687-92.
23. . ASMBS. Studies weigh in on safety and effectiveness of newer bariatric and metabolic surgery procedure. Available from: https://asmbs.org/resources/studies-weigh-in-on-safety-and- [Last accessed on 12 Apr 2022]
24. Behary J, Kumbhari V. Advances in the endoscopic management of obesity. Gastroenterol Res Pract 2015;2015:757821.
25. Ponce J, Woodman G, Swain J, et al. REDUCE Pivotal Trial Investigators. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis 2015;11:874-81.
26. Singh S, Hourneaux de Moura DT, Khan A, et al. Safety and efficacy of endoscopic sleeve gastroplasty worldwide for treatment of obesity: a systematic review and meta-analysis. Surg Obes Relat Dis 2020;16:340-51.
27. Boules M, Chang J, Haskins IN, et al. Endoscopic management of post-bariatric surgery complications. World J Gastrointest Endosc 2016;8:591-9.
28. Spaw AT, Husted JD. Bleeding after laparoscopic gastric bypass: Case report and literature review. Surg Obes Relat Dis 2005;1:99-103.
29. Kaplan LM. Gastrointestinal management of the bariatric surgery patient. Gastroenterol Clin North Am 2005;34:105-25.
30. Bellorin O, Abdemur A, Sucandy I, Szomstein S, Rosenthal RJ. Understanding the significance, reasons and patterns of abnormal vital signs after gastric bypass for morbid obesity. Obes Surg 2011;21:707-13.
31. Ferreira LE, Song LM, Baron TH. Management of acute postoperative hemorrhage in the bariatric patient. Gastrointest Endosc Clin N Am 2011;21:287-94.
32. Rao AD, Ramalingam G. Exsanguinating hemorrhage following gastric erosion after laparoscopic adjustable gastric banding. Obes Surg 2006;16:1675-8.
33. Fridman A, Szomstein S, Rosenthal RJ. Postoperative bleeding in the bariatric surgery patient. The ASMBS Textbook of Bariatric Surgery 2015;21:241-7.
34. Nguyen NT, Brethauer SA, Morton JM, Ponce J, Rosenthal RJ. The ASMBS textbook of bariatric surgery. Available from: https://link.springer.com/book/10.1007/978-3-030-27021-6?noAccess=true [Last accessed on 12 Apr 2022].
35. Córdova H, Fernández-esparrach G. Treatment of bleeding after bariatric surgery. Techniques in Gastrointestinal Endoscopy 2010;12:130-5.
36. Kumar N, Thompson CC. Endoscopic management of complications after gastrointestinal weight loss surgery. Clin Gastroenterol Hepatol 2013;11:343-53.
37. Cappell MS, Friedel D. Initial management of acute upper gastrointestinal bleeding: from initial evaluation up to gastrointestinal endoscopy. Med Clin North Am 2008;92:491-509, xi.
38. Baradarian R, Ramdhaney S, Chapalamadugu R, et al. Early intensive resuscitation of patients with upper gastrointestinal bleeding decreases mortality. Am J Gastroenterol 2004;99:619-22.
39. Heneghan HM, Meron-Eldar S, Yenumula P, et al. Incidence and management of bleeding complications after gastric bypass surgery in the morbidly obese. Surg Obes Relat Dis 2012;8:729-35.
40. Campos JM, Moon R, Teixeira A, et al. Endoscopic management of massive hemorrhage 12 h post laparoscopic Roux-en-Y gastric bypass. Obes Surg 2015;25:1981-3.
41. Mehran A, Szomstein S, Zundel N, Rosenthal R. Management of acute bleeding after laparoscopic Roux-en-Y gastric bypass. Obes Surg 2003;13:842-7.
42. Lim R, Beekley A, Johnson DC, Davis KA. Early and late complications of bariatric operation. Trauma Surg Acute Care Open 2018;3:e000219.
43. Rahman R, Nguyen DL, Sohail U, et al. Pre-endoscopic erythromycin administration in upper gastrointestinal bleeding: an updated meta-analysis and systematic review. Ann Gastroenterol 2016;29:312-7.
44. Eisendrath P, Deviere J. Major complications of bariatric surgery: endoscopy as first-line treatment. Nat Rev Gastroenterol Hepatol 2015;12:701-10.
45. Huang CS, Farraye FA. Endoscopy in the bariatric surgical patient. Gastroenterol Clin North Am 2005;34:151-66.
46. Kumbhari V, Cai JX, Schweitzer MA. Endoscopic management of bariatric surgical complications. Curr Opin Gastroenterol 2015;31:359-67.
47. Moretto M, Mottin CC, Padoin AV, Berleze D, Repetto G. Endoscopic management of bleeding after gastric bypass -- a therapeutic alternative. Obes Surg 2004;14:706.
48. Tang SJ, Rivas H, Tang L, et al. Endoscopic hemostasis using endoclip in early gastrointestinal hemorrhage after gastric bypass surgery. Obes Surg 2007;17:1261-7.
49. Issa H, Al-Saif O, Al-Momen S, Bseiso B, Al-Salem A. Bleeding duodenal ulcer after Roux-en-Y gastric bypass surgery: the value of laparoscopic gastroduodenoscopy. Ann Saudi Med 2010;30:67-9.
50. Gill KR, McKinney JM, Stark ME, Bouras EP. Investigation of the excluded stomach after Roux-en-Y gastric bypass: the role of percutaneous endoscopy. World J Gastroenterol 2008;14:1946-8.
51. Anjiki H, Kamisawa T, Sanaka M, Ishii T, Kuyama Y. Endoscopic hemostasis techniques for upper gastrointestinal hemorrhage: a review. World J Gastrointest Endosc 2010;2:54-60.
52. Valli PV, Gubler C. Review article including treatment algorithm: endoscopic treatment of luminal complications after bariatric surgery. Clin Obes 2017;7:115-22.
53. Chuttani R, Barkun A, Carpenter S, et al. Technology assessment committee. Endoscopic clip application devices. Gastrointest Endosc 2006;63:746-50.
54. Jensen DM, Machicado GA, Hirabayashi K. Randomized controlled study of 3 different types of hemoclips for hemostasis of bleeding canine acute gastric ulcers. Gastrointest Endosc 2006;64:768-73.
55. Siegal SR, Pauli EM. Endoscopic management of postoperative complications. Surg Clin North Am 2020;100:1115-31.
56. Hachisu T, Miyazaki S, Hamaguchi K. Endoscopic clip-marking of lesions using the newly developed HX-3L clip. Surg Endosc 1989;3:142-7.
57. Vanbiervliet G, Filippi J, Karimdjee BS, et al. The role of clips in preventing migration of fully covered metallic esophageal stents: a pilot comparative study. Surg Endosc 2012;26:53-9.
58. Tang SJ, Tang L, Gupta S, Rivas H. Endoclip closure of jejunal perforation after balloon dilatation. Obes Surg 2007;17:540-3.
59. Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc 2006;63:596-601.
60. Lin HJ, Hsieh YH, Tseng GY, et al. A prospective, randomized trial of large- versus small-volume endoscopic injection of epinephrine for peptic ulcer bleeding. Gastrointest Endosc 2002;55:615-9.
61. Marmo R, Rotondano G, Piscopo R, et al. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol 2007;102:279-89; quiz 469.
62. Vergara M, Calvet X, Gisbert JP, Vergara M. Epinephrine injection versus epinephrine injection and a second endoscopic method in high risk bleeding ulcers. Cochrane Database Syst Rev ;2014:CD005584.
63. Lo CC, Hsu PI, Lo GH, et al. Comparison of hemostatic efficacy for epinephrine injection alone and injection combined with hemoclip therapy in treating high-risk bleeding ulcers. Gastrointest Endosc 2006;63:767-73.
64. Shi K, Shen Z, Zhu G, Meng F, Gu M, Ji F. Systematic review with network meta-analysis: dual therapy for high-risk bleeding peptic ulcers. BMC Gastroenterol 2017;17:55.
65. Kirschniak A, Kratt T, Stüker D, et al. A new endoscopic over-the-scope clip system for treatment of lesions and bleeding in the GI tract: first clinical experiences. Gastrointest Endosc 2007;66:162-7.
66. Farnik H, Driller M, Kratt T, et al. Indication for “Over the scope” (OTS)-clip vs. covered self-expanding metal stent (cSEMS) is unequal in upper gastrointestinal leakage: results from a retrospective head-to-head comparison. PLoS One 2015;10:e0117483.
67. Schmidt A, Gölder S, Goetz M, et al. Over-the-scope clips are more effective than standard endoscopic therapy for patients with recurrent bleeding of peptic ulcers. Gastroenterology 2018;155:674-686.e6.
68. Bartell N, Bittner K, Kaul V, Kothari TH, Kothari S. Clinical efficacy of the over-the-scope clip device: a systematic review. World J Gastroenterol 2020;26:3495-516.
69. Wedi E, Gonzalez S, Menke D, et al. One hundred and one over-the-scope-clip applications for severe gastrointestinal bleeding, leaks and fistulas. World J Gastroenterol 2016;22:1844-53.
70. Wedi E, von Renteln D, Gonzalez S, et al. Use of the over-the-scope-clip (OTSC) in non-variceal upper gastrointestinal bleeding in patients with severe cardiovascular comorbidities: a retrospective study. Endosc Int Open 2017;5:E875-82.
71. Manta R, Mangiafico S, Zullo A, et al. First-line endoscopic treatment with over-the-scope clips in patients with either upper or lower gastrointestinal bleeding: a multicenter study. Endosc Int Open 2018;6:E1317-21.
72. Manno M, Mangiafico S, Caruso A, et al. First-line endoscopic treatment with OTSC in patients with high-risk non-variceal upper gastrointestinal bleeding: preliminary experience in 40 cases. Surg Endosc 2016;30:2026-9.
74. Chahal D, Lee JGH, Ali-Mohamad N, Donnellan F. High rate of re-bleeding after application of hemospray for upper and lower gastrointestinal bleeds. Dig Liver Dis 2020;52:768-72.
75. Chahal D, Sidhu H, Zhao B, et al. Efficacy of hemospray (TC-325) in the treatment of gastrointestinal bleeding: an updated systematic review and meta-analysis. J Clin Gastroenterol 2021;55:492-8.
76. Kantsevoy SV, Thuluvath PJ. Successful closure of a chronic refractory gastrocutaneous fistula with a new endoscopic suturing device (with video). Gastrointest Endosc 2012;75:688-90.
77. Armengol-Miro JR, Dot J, Abu-Suboh Abadia M, et al. New endoscopic suturing device for closure of chronic gastrocutaneous fistula in an immunocompromised patient. Endoscopy 2011;43 Suppl 2 UCTN:E403-4.
78. Kantsevoy SV, Bitner M, Davis JM, et al. Endoscopic suturing closure of large iatrogenic colonic perforation. Gastrointest Endosc 2015;82:754-5.
79. Kantsevoy SV, Bitner M, Hajiyeva G, et al. Endoscopic management of colonic perforations: clips versus suturing closure (with videos). Gastrointest Endosc 2016;84:487-93.
80. Kumar N, Thompson CC. A novel method for endoscopic perforation management by using abdominal exploration and full-thickness sutured closure. Gastrointest Endosc 2014;80:156-61.
81. Bonin EA, Wong Kee Song LM, et al. Closure of a persistent esophagopleural fistula assisted by a novel endoscopic suturing system. Endoscopy 2012;44 Suppl 2 UCTN:E8-9.
82. Chon SH, Toex U, Plum PS, et al. Successful closure of a gastropulmonary fistula after esophagectomy using the apollo overstitch and endoscopic vacuum therapy. Endoscopy 2018;50:E149-50.
83. Bergström M, Swain P, Park PO. Early clinical experience with a new flexible endoscopic suturing method for natural orifice transluminal endoscopic surgery and intraluminal endosurgery (with videos). Gastrointest Endosc 2008;67:528-33.
84. Agarwal A, Benias P, Brewer Gutierrez OI, et al. Endoscopic suturing for management of peptic ulcer-related upper gastrointestinal bleeding: a preliminary experience. Endosc Int Open 2018;6:E1439-44.
85. Barola S, Magnuson T, Schweitzer M, et al. Endoscopic suturing for massively bleeding marginal ulcer 10 days post Roux-en-Y gastric bypass. Obes Surg 2017;27:1394-6.
87. Cheriyan T, Maier SP 2nd, Bianco K, et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J 2015;15:752-61.
88. Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW. A prospective double-blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty 2013;28:78-82.
89. Lin SY, Chen CH, Fu YC, et al. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty 2015;30:776-80.
90. Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23-32.
91. Chakravartty S, Sarma DR, Chang A, Patel AG. Staple line bleeding in sleeve gastrectomy-a simple and cost-effective solution. Obes Surg 2016;26:1422-8.
92. Hussain A, Al-Shoek I, El-Hasani S. The use of tranexamic acid in sleeve gastrectomy. Obes Surg 2017;27:198-9.
93. Klaassen RA, Selles CA, van den Berg JW, Poelman MM, van der Harst E. Tranexamic acid therapy for postoperative bleeding after bariatric surgery. BMC Obes 2018;5:36.
94. Roberts I, Shakur-still H, Afolabi A, et al. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet 2020;395:1927-36.
95. Karadaş A, Doğan NÖ, Pinar SG, et al. A randomized controlled trial of the effects of local tranexamic acid on mortality, rebleeding, and recurrent endoscopy need in patients with upper gastrointestinal hemorrhage. Eur J Gastroenterol Hepatol 2020;32:26-31.
96. Tavakoli N, Mokhtare M, Agah S, et al. Comparison of the efficacy of intravenous tranexamic acid with and without topical administration versus placebo in urgent endoscopy rate for acute gastrointestinal bleeding: a double-blind randomized controlled trial. United European Gastroenterol J 2018;6:46-54.
97. Hawkey GM, Cole AT, McIntyre AS, Long RG, Hawkey CJ. Drug treatments in upper gastrointestinal bleeding: value of endoscopic findings as surrogate end points. Gut 2001;49:372-9.
98. Holstein CC, Eriksson SB, Källén R. Tranexamic acid as an aid to reducing blood transfusion requirements in gastric and duodenal bleeding. Br Med J (Clin Res Ed) 1987;294:7-10.
99. Barer D, Ogilvie A, Henry D, et al. Cimetidine and tranexamic acid in the treatment of acute upper-gastrointestinal-tract bleeding. N Engl J Med 1983;308:1571-5.
100. Bergqvist D, Dahlgren S, Hessman Y. Local inhibition of the fibrinolytic system in patients with massive upper gastrointestinal hemorrhage. Ups J Med Sci 1980;85:173-8.
101. Engqvist A, Broström O, von Feilitzen F, et al. Tranexamic acid in massive haemorrhage from the upper gastrointestinal tract: a double-blind study. Scand J Gastroenterol 1979;14:839-44.
102. Biggs JC, Hugh TB, Dodds AJ. Tranexamic acid and upper gastrointestinal haemorrhage--a double-blind trial. Gut 1976;17:729-34.
103. Cormack F, Jouhar A, Chakrabarti R, Fearnley G. Tranexamic acid in upper gastrointestinal hæmorrhage. Lancet 1973;301:1207-8.
104. Gluud LL, Klingenberg SL, Langholz E, Gluud LL. Tranexamic acid for upper gastrointestinal bleeding. Cochrane Database Syst Rev 2014;2014:CD006640.
105. Kamal F, Khan MA, Lee-Smith W, et al. Efficacy and safety of tranexamic acid in acute upper gastrointestinal bleeding: meta-analysis of randomised controlled trials. Scand J Gastroenterol 2020;55:1390-7.
106. Odutayo A, Desborough MJR, Trivella M, et al. Restrictive versus liberal blood transfusion for gastrointestinal bleeding: a systematic review and meta-analysis of randomised controlled trials. The Lancet Gastroenterology & Hepatology 2017;2:354-60.
107. Rosenthal RJ, Szomstein S, Kennedy CI, Soto FC, Zundel N. Laparoscopic surgery for morbid obesity: 1,001 consecutive bariatric operations performed at the bariatric institute, Cleveland Clinic Florida. Obes Surg 2006;16:119-24.
108. Chousleb E, Szomstein S, Podkameni D, et al. Routine abdominal drains after laparoscopic Roux-en-Y gastric bypass: a retrospective review of 593 patients. Obes Surg 2004;14:1203-7.
109. Scheirey CD, Scholz FJ, Shah PC, et al. Radiology of the laparoscopic Roux-en-Y gastric bypass procedure: conceptualization and precise interpretation of results. Radiographics 2006;26:1355-71.
110. Simillis C, Fachiri M, Bonanomi G. A challenging gastrointestinal hemorrhage after gastric bypass treated with interventional radiology. Surg Obes Relat Dis 2016;12:e59-62.
111. Mala T, Søvik TT, Schou CF, Kristinsson J. Blood clot obstruction of the jejunojejunostomy after laparoscopic gastric bypass. Surg Obes Relat Dis 2013;9:234-7.
112. Awais O, Raftopoulos I, Luketich JD, Courcoulas A. Acute, complete proximal small bowel obstruction after laparoscopic gastric bypass due to intraluminal blood clot formation. Surg Obes Relat Dis 2005;1:418-22; discussion 422.
113. Koppman JS, Li C, Gandsas A. Small bowel obstruction after laparoscopic Roux-en-Y gastric bypass: a review of 9,527 patients. J Am Coll Surg 2008;206:571-84.
114. Jossart GH. Complications of sleeve gastrectomy: bleeding and prevention. Surg Laparosc Endosc Percutan Tech 2010;20:146-7.
115. Choi YY, Bae J, Hur KY, Choi D, Kim YJ. Reinforcing the staple line during laparoscopic sleeve gastrectomy: does it have advantages? Obes Surg 2012;22:1206-13.
116. Janik MR, Walędziak M, Brągoszewski J, Kwiatkowski A, Paśnik K. Prediction model for hemorrhagic complications after laparoscopic sleeve gastrectomy: development of sleeve bleed calculator. Obes Surg 2017;27:968-72.
117. Sabry K, Hamed A, Habib H, Helmy M, Aboizeid. Management of acute bleeding post laparoscopic sleeve gastrectomy. Available from: https://www.omicsonline.org/open- [Last accessed on 12 Apr 2022].
118. da Rocha LC, Ayub Pérez OA, Arantes V. Endoscopic management of bariatric surgery complications: what the gastroenterologist should know. Rev Gastroenterol Mex 2016;81:35-47.
119. Richardson JF, Lee JG, Smith BR, et al. Laparoscopic transgastric endoscopy after roux-en-y gastric bypass: case series and review of the literature. Am Surg 2012;78:1182-6.
120. BE-SAFE. Bariatric endoscopy- skill acquisition focused evaluation Available from: https://besafe.asmbs.org/ [Last accessed on 12 Apr 2022].
121. Guerrier JB, Mehaffey JH, Schirmer BD, Hallowell PT. Reinforcement of the staple line during gastric sleeve: a comparison of buttressing or oversewing, versus no reinforcement- a single-institution study. Am Surg 2018;84:690-4.
122. Borjas G, Gonzalez M, Maldonado A, Urdaneta A, Ramos E. Oversewing staple line of the gastric remnant in gastric bypass reduces postoperative bleeding. Ann Med Surg (Lond) 2021;67:102534.
123. Abellán I, López V, Lujan J, et al. Stapling versus hand suture for gastroenteric anastomosis in Roux-en-Y gastric bypass: a randomized clinical trial. Obes Surg 2015;25:1796-801.
124. Fakas S, Elias M, Lim D, Meytes V. Comparison of gastrojejunostomy techniques and anastomotic complications: a systematic literature review. Surg Endosc 2021;35:6489-96.
125. Rabl C, Peeva S, Prado K, et al. Early and late abdominal bleeding after Roux-en-Y gastric bypass: sources and tailored therapeutic strategies. Obes Surg 2011;21:413-20.
126. Braley SC, Nguyen NT, Wolfe BM. Late gastrointestinal hemorrhage after gastric bypass. Obes Surg 2002;12:404-7.
127. Madan AK, DeArmond G, Ternovits CA, Beech DJ, Tichansky DS. Laparoscopic revision of the gastrojejunostomy for recurrent bleeding ulcers after past open revision gastric bypass. Obes Surg 2006;16:1662-8.
128. Antúnez DJ, Fernández NC, Serrano JO. Unusual case of upper gastrointestinal bleeding after laparoscopic gastric bypass: erosion of gastric remnant involving a diaphragmatic vessel. Surg Obes Relat Dis 2011;7:328-9.
129. Zerey M, Sigmon LB, Kuwada TS, Heniford BT, Sing RF. Bleeding duodenal ulcer after roux-en-Y gastric bypass surgery. J Am Osteopath Assoc 2008;108:25-7.
130. Gupta A, Shah MM, Kalaskar SN, Kroh M. Late postoperative bleeding after Roux-en-Y gastric bypass: management and review of literature. BMJ Case Rep 2018;11:e226271.
131. Goel R, Nasta AM, Goel M, et al. Complications after bariatric surgery: a multicentric study of 11,568 patients from Indian bariatric surgery outcomes reporting group. J Minim Access Surg 2021;17:213-20.
132. Rasmussen JJ, Fuller W, Ali MR. Marginal ulceration after laparoscopic gastric bypass: an analysis of predisposing factors in 260 patients. Surg Endosc 2007;21:1090-4.
133. Maclean LD, Rhode BM, Nohr C, Katz S, Mclean AH. Stomal ulcer after gastric bypass11An editorial to this article can be found on page 87. J Am Coll Surg 1997;185:1-7.
134. Capella JF, Capella RF. Gastro-gastric fistulas and marginal ulcers in gastric bypass procedures for weight reduction. Obes Surg 1999;9:22-7; discussion 28.
135. Capella JF, Capella RF. Staple disruption and marginal ulceration in gastric bypass procedures for weight reduction. Obes Surg 1996;6:44-9.
136. Gill RS, Whitlock KA, Mohamed R, et al. The role of upper gastrointestinal endoscopy in treating postoperative complications in bariatric surgery. J Interv Gastroenterol 2012;2:37-41.
137. Azagury DE, Abu Dayyeh BK, Greenwalt IT, Thompson CC. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy 2011;43:950-4.
138. Kitamura RK. The Management of GI bleeding after gastric bypass surgery. Int J Surg Res Pract 2015;2:2. Available from:
139. Puri V, Alagappan A, Rubin M, Merola S. Management of bleeding from gastric remnant after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2012;8:e3-5.
140. Ross AS, Semrad C, Alverdy J, Waxman I, Dye C. Use of double-balloon enteroscopy to perform PEG in the excluded stomach after Roux-en-Y gastric bypass. Gastrointest Endosc 2006;64:797-800.
141. Silecchia G, Catalano C, Gentileschi P, et al. Virtual gastroduodenoscopy: a new look at the bypassed stomach and duodenum after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg 2002;12:39-48.
142. Sakai P, Kuga R, Safatle-Ribeiro AV, et al. Is it feasible to reach the bypassed stomach after Roux-en-Y gastric bypass for morbid obesity? Endoscopy 2005;37:566-9.
143. Kuga R, Safatle-Ribeiro AV, Faintuch J, et al. Endoscopic findings in the excluded stomach after Roux-en-Y gastric bypass surgery. Arch Surg 2007;142:942-6.
144. Coblijn UK, Lagarde SM, de Castro SM, et al. The influence of prophylactic proton pump inhibitor treatment on the development of symptomatic marginal ulceration in Roux-en-Y gastric bypass patients: a historic cohort study. Surg Obes Relat Dis 2016;12:246-52.
145. Ying VW, Kim SH, Khan KJ, et al. Prophylactic PPI help reduce marginal ulcers after gastric bypass surgery: a systematic review and meta-analysis of cohort studies. Surg Endosc 2015;29:1018-23.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Giannopoulos S, Pokala B, Stefanidis D. Management of gastrointestinal bleeding following bariatric surgery. Mini-invasive Surg 2022;6:22. http://dx.doi.org/10.20517/2574-1225.2021.135
AMA Style
Giannopoulos S, Pokala B, Stefanidis D. Management of gastrointestinal bleeding following bariatric surgery. Mini-invasive Surgery. 2022; 6: 22. http://dx.doi.org/10.20517/2574-1225.2021.135
Chicago/Turabian Style
Giannopoulos, Spyridon, Bhavani Pokala, Dimitrios Stefanidis. 2022. "Management of gastrointestinal bleeding following bariatric surgery" Mini-invasive Surgery. 6: 22. http://dx.doi.org/10.20517/2574-1225.2021.135
ACS Style
Giannopoulos, S.; Pokala B.; Stefanidis D. Management of gastrointestinal bleeding following bariatric surgery. Mini-invasive. Surg. 2022, 6, 22. http://dx.doi.org/10.20517/2574-1225.2021.135
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 79 clicks
Cite This Article 79 clicks


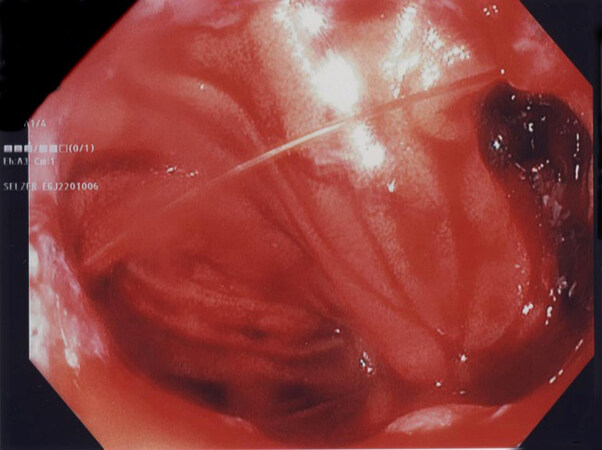
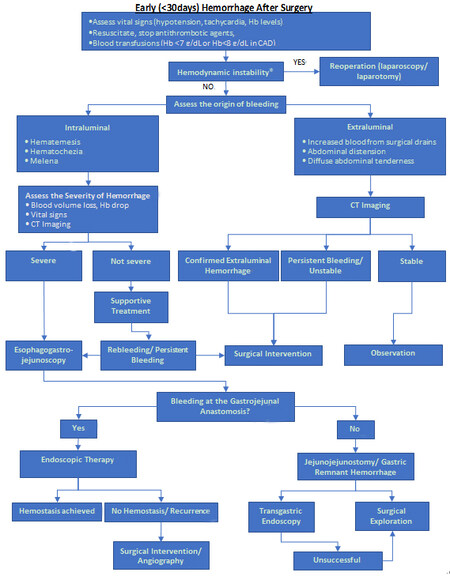
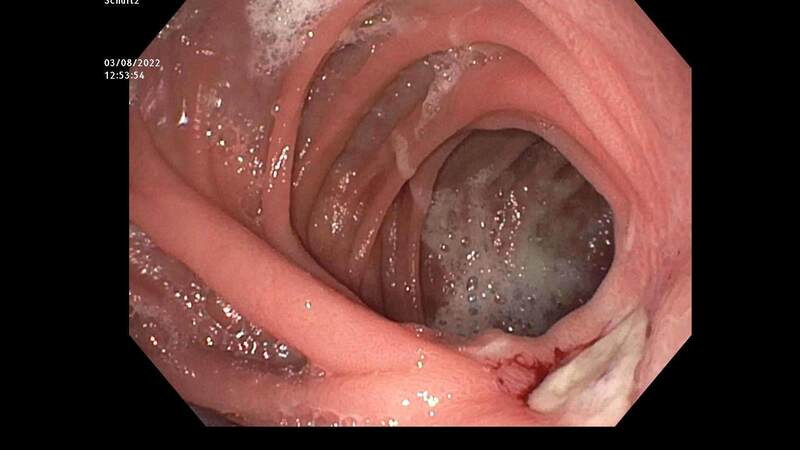
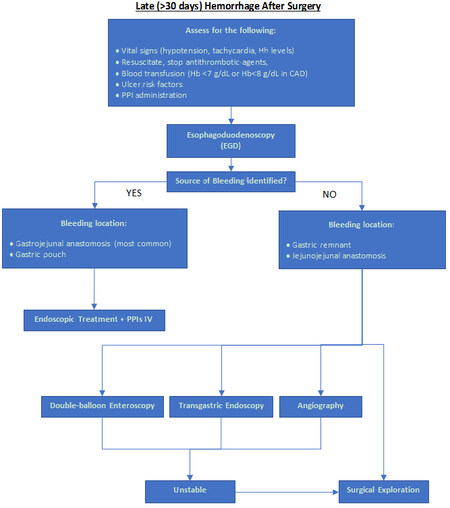









Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.