Endoscopic submucosal dissection for early gastric adenocarcinoma: basic procedures and management of complications
Abstract
Mastery of endoscopic submucosal dissection (ESD) requires a deep understanding of not only the technique but also the preparation, electrosurgical unit, its peripherals, and probable procedural complications. An endoscope with a water-jet function is suitable for gastric ESD. A transparent attachment on the tip of the endoscope is necessary. The mucosal preparation is important for proper lesion recognition and precise device movement during the procedure and to reduce the risk of complications. A needle-type knife allows accurate tissue dissection and is especially useful for dissecting fibrosis in the submucosa. A partially insulated-type knife prevents current transmission from the tip of the needle to the deep tissues, thus reducing the risk of unintentional perforation or bleeding. When using a scissor-type knife, stable device movement is possible and unintended tissue dissection is minimized. The electrosurgical unit mode must be adjusted according to the different tissue conditions. The basic techniques for ESD incision and dissection should be mastered, and the appropriate knives, accessories, strategy, and traction device should be methodically selected by each operator. The main complications of ESD are bleeding and perforation, which should be treated appropriately when they occur.
Keywords
INTRODUCTION
Endoscopic submucosal dissection (ESD) is currently a standard treatment for intramucosal neoplasia in the stomach. The ESD provides about ten times higher en bloc resection rate (92.4% vs. 51.7%, OR: 9.69), five times higher histological complete resection rate (82.1% vs. 42.2%, OR: 5.66), and one tenth recurrence rate (0.76% vs. 6.4%, OR: 0.10) than endoscopic mucosal resection (EMR)[1]. The effectiveness of ESD for the treatment of EGC patients has been proven by good long-term outcomes in multicenter prospective studies[2,3].
Despite excellent published outcomes, there is still a paucity of details about the basic concept and technique of the ESD procedures. In this article, we impart practical information necessary for the proper execution of the technique based on our experiences and currently available knowledge. We believe an advanced procedure is accomplished with the accumulation of solid basic procedures.
PREPARATION
Equipment
Endoscopes
An endoscope with a small bending radius and a water-jet function is suitable for gastric ESD. A wide working channel diameter of ≥ 3.2 mm, for example, the GIF-Q260J, GIF-H290T, GIF-1TH190 (all by Olympus, Co., Ltd.), or EG-L580RD7 (Fujifilm Medical Systems, Co., Ltd.), is preferred because, in the case of bleeding, water or air can be suctioned while the hemostatic devices are kept inserted in the working channel.
If the lesion is located at the fornix, the gastric angle, or the anterior wall of the lower corpus, where the endoscopic approach is difficult, a multibending video endoscope such as the GIF-2TQ260M (Olympus, Co., Ltd.) is useful. The scope has proximal two-way bending in addition to distal four-way deflection.
Transparent attachment
For gastric ESD, a transparent attachment on the tip of the endoscope is necessary. During mucosal incision, the attachment stabilizes the operative field and applies adequate tension to the target mucosa. In submucosal dissection, the dissected specimen is lifted, and working space is created for continuous submucosal dissection. In the case of bleeding, it can separate surrounding tissue around the area of bleeding and facilitate the identification of the bleeding point. Because each device has a different appropriate working distance, the protrusion length of the attachment must be adjusted. Usually, a long attachment with a protrusion length of 4-6 mm is selected for the needle-type knife or scissor-type knife, while a short attachment with a protrusion length of 2 mm is often selected for the insulated tip-type knife. In cases of severe fibrosis or poor accessibility to the submucosal space is expected, and the tunneling method is selected, a tapered-tip attachment, such as the ST hood (Fujifilm, Co., Ltd.), would be useful.
Insufflation gas
Two meta-analyses indicated that CO2 insufflation significantly reduced post-procedural abdominal pain but did not affect other clinical outcomes such as en bloc resection rate, procedure time, incidence of adverse events, etc. of gastric ESD[4,5]. The influence of the type of insufflated gas may be less significant than that of colonic or esophageal ESD. However, CO2 insufflation is advantageous over air insufflation in the case of unexpected complications, especially perforation.
Mucosal preparation
Poor mucosal visibility, caused by mucus or bubbles, can interfere with proper lesion recognition and precise device movement during the procedure. Moreover, when complications such as perforation or bleeding occur, inadequate mucosal preparation makes their management difficult. In our institution, a mixture comprised of 20,000 units of pronase, 1 g of sodium bicarbonate, 20 mg of dimethylpolysiloxane, and 80 mL of water is administered before ESD.
Sedation
In Japanese practice, most gastric ESD procedures are performed in the endoscopy unit under deep sedation using a combination of intravenous benzodiazepines, i.e. midazolam, diazepam, flunitrazepam, etc., and analgesics, i.e. pethidine, pentazocine, etc. This is because ESD usually develops from EMR, and the same setting can be continued. In the case of patients being restless, haloperidol drip infusion or continuous infusion of dexmedetomidine is useful. When an extremely long (> 3 h) procedure is expected or for lesions in difficult locations, propofol sedation or general anesthesia could be considered. With deeper sedation or under general anesthesia, a patient’s movement disappears, and an operator can concentrate on the procedure.
DEVICES
Endo-knife
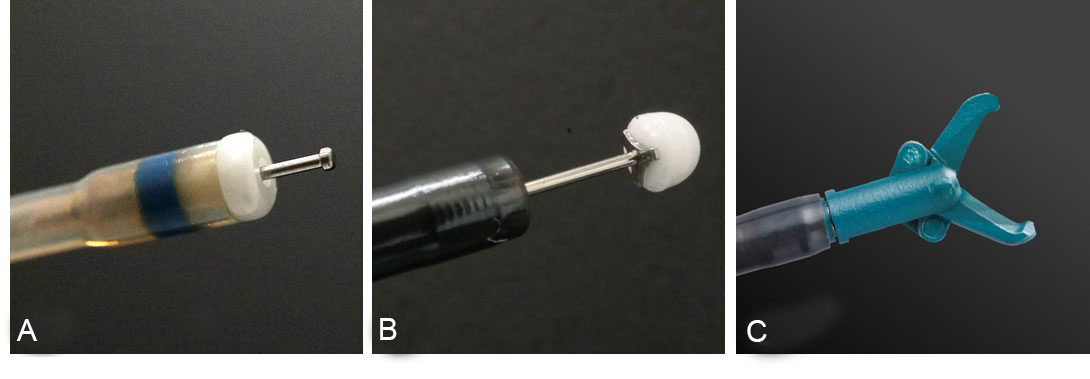
There are several types of knives available for gastric ESD, and they are mainly grouped into needle-type, partially insulated-type, and scissor-type knives [Figure 1].
Figure 1. Endo-knives for endoscopic submucosal dissection (ESD): (A) needle-type knife (Dual Knife J. Olympus, Co., Ltd.); (B) partially insulated-type knife (IT Knife2. Olympus, Co., Ltd.); and (C) scissor-type knife (SB Knife Jr. Sumitomo Bakelite, Co., Ltd.). The image in (C) is used with permission from Sumitomo Bakelite, Co., Ltd.
Needle-type knife
A needle-type knife can be used in all situations in ESD procedures, that is, mucosal marking, mucosal incision, and submucosal dissection. The needle tip allows accurate and pin-point tissue dissection and is especially useful for dissecting fibrosis in the submucosa. Although the needle tip enables the tissue to be incised in any direction, the chance of perforation is higher than that of other knives. Therefore, precise adjustment of the depth and direction of movement is required for a safe operation. Recently, most needle-type knives have been equipped with a water-jet function, which allows submucosal injection by knives. This increases safety and reduces procedural time.
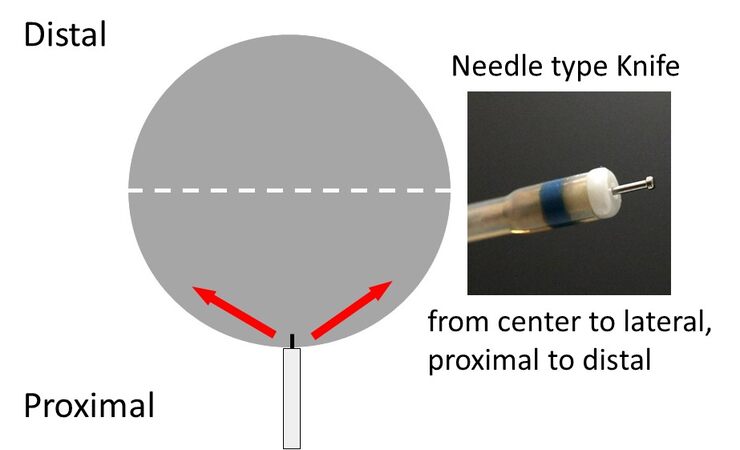
In general, the direction of movement for mucosal incision and submucosal dissection with a needle knife-type device is from the center to the periphery and from the near side to the far side [Figure 2].
Figure 2. Direction of movement for mucosal incision and submucosal dissection with a needle-type knife.
Representative needle knife-type devices are the Dual Knife (Olympus, Co., Ltd.), Flush Knife (Fujifilm, Co., Ltd.), Splash M-Knife (Pentax, Co., Ltd.), Hybrid Knife (ERBE, Co., Ltd.), and ORISE ProKnife (Boston Scientific, Co., Ltd.).
Partially insulated-type knife
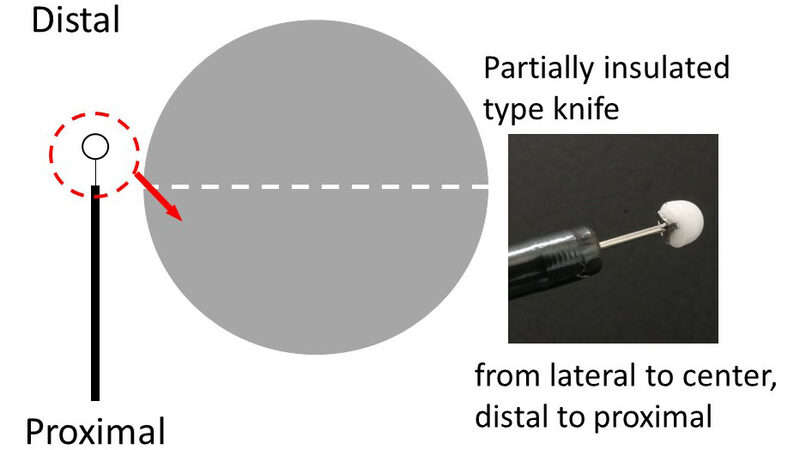
A partially insulated-type knife has a small ceramic ball at the tip of the needle to avoid electrical cautery conduction. The ceramic tip prevents current transmission from the tip of the needle to the deep tissues, thus reducing the risk of unintentional perforation or bleeding. Because the non-insulated tip cannot dissect the tissue, the proximal metal part is used for tissue dissection. The characteristic structure of the insulated-type knife requires the use of a needle-type knife for marking and pre-cutting. In general, the direction of movement for mucosal incision and submucosal dissection with an insulated-type knife is contrary to that of the needle-type knife: from the periphery to the center, and from the far side to the near side [Figure 3].
Figure 3. Direction of movement for mucosal incision and submucosal dissection with a partially insulated-type knife.
Because the axes of the scope tip and knife are aligned, a unique scope position is required to make the scope tip parallel to the target. The blade is 4 mm long, so the amount of tissue that can be dissected simultaneously is large. Examples of partially insulated-type devices are the insulated-tip (IT) knife 2 (Olympus, Co., Ltd.), SAFE knife (Fujifilm, Co., Ltd.), and Mucosectom (Pentax, Co., Ltd.).
Scissor-type knife
The scissor-type knife has monopolar scissor blades covered with an insulated coating element on the external side and can be rotated 360°. To use the scissor-type knife, the target tissue is first grasped, slightly pulled up, and an electrical current is applied. As the tissue is grasped by the device before cutting, the device is stabilized; hence, unintended tissue dissection is minimized. Furthermore, vessels in the dissecting tissue are also grasped and prophylactically cauterized; therefore, bleeding during submucosal dissection is rare. One disadvantage of the scissor-type knife is the prerequisite for a trained assistant as the assistant is responsible for the rotation of the knife according to the direction of the submucosa. Examples of scissor-type knives are the Clutch Cutter (Fujifilm, Co., Ltd.) and Stag-Beetle (SB) knife (Sumitomo Bakelite, Co., Ltd.).
Endo-knife with water-jet function
Some knives are equipped with a water-jet function, enabling surface mucosal washing and additional submucosal fluid injection. A randomized controlled trial (RCT) demonstrated the needle-type knife with water-jet function significantly shortened the procedure time of colorectal ESD because of the reduction of the number of device exchanges for submucosal injection[6,7]. Representative endo-knives with a water-jet function are the Flush knife (Fujifilm, Co., Ltd.), Hybrid knife (ERBE, Co. Ltd.), Dual knife-J (Olympus, Co., Ltd.), Hook Knife-J (Olympus, Co., Ltd.), and Triangle Tip knife J (Olympus, Co., Ltd.).
Hemostatic Devices
Bleeding commonly occurs during gastric ESD. Because bleeding interrupts ESD procedures, for instance, tissue cutting and dissection, it must be controlled as soon as possible. Minor bleeding can be managed with ESD knives using the coagulation mode; however, severe bleeding requires a hemostatic device for hemostasis.
Hemostatic forceps
The cup of the hemostatic forceps is smaller than that of the conventional hot biopsy forceps to precisely grasp tissue-containing vessels. The tip of the hemostatic forceps is rotatable such that the direction of the cup can be adjusted according to the shape of the bleeding vessel or the surrounding tissue. There are two types of hemostatic forceps: monopolar and bipolar. The former includes the Coagrasper (Olympus, Co., Ltd.) and Raicho (KANEKA, Co., Ltd.), and the latter includes Hemostat Y (Pentax, Co., Ltd.). With bipolar forceps, only the tissue between the cups is cauterized, thus hemostasis can be instantly achieved with low power, while the thermal effect is contained without spreading to the surrounding tissues. Bipolar forceps are suitable for organs with thinner submucosal layers such as the esophagus or colon, because the thermal effect does not penetrate deep into the tissue; however, the thermal effect may be too weak to cauterize the thick arteries in the deep mucosa and submucosa of the stomach.
Hot biopsy forceps
Hot biopsy forceps are used for hemostasis during gastric ESD. They feature larger cups than hemostatic forceps; hence, they can capture more tissue and cauterize wider and deeper areas. This property is suitable for ESD of the gastric body or cardia, where thick arteries often exist. An example of hot biopsy forceps is the Radial Jaw 4 (Boston Scientific, Co., Ltd.).
Electrosurgical unit setting and device maneuver
Appropriate settings for an electrosurgical unit (ESU) and basic knowledge of electrosurgery have a remarkable impact on the success of gastric ESD. Widely used electrosurgical units are VIO 200/300 or VIO 3 (ERBE, Co., Ltd.) and ESG-100/300 (Olympus, Co., Ltd.).
The ESU mode and device maneuverability (control of the contact area) must be adjusted according to the different tissue conditions. The key tissue conditions are: (1) the rigidity of the tissue due to collagen fibers; and (2) the presence of vessels. Rigid tissue must be incised with the cut mode (AUTO CUT, ENDO CUT, etc.). Soft tissue can be cut in either cut mode or coagulation mode (FORCED COAG, SWIFT COAG, etc.). Dissection of the soft tissue with cut mode avoids tissue shrinkage and maintains a clear-cutting plane while dissection with coagulation mode prevents bleeding when blood vessels are contained. Bleeding interferes with the visualization of the operation field and worsens electrical conduction, thus interrupting the ESD procedure. Therefore, it is better to dissect the soft tissue containing blood vessels in coagulation mode to avoid bleeding. Although thin vessels can be coagulated without bleeding by the ESD knives, when a thick vessel is seen in the tissue, it is better to use hemostatic forceps with SOFT COAG mode to coagulate the vessel. When the coagulation mode is used for dissection of the soft tissue without vessels, the contact area between the tissue and the knife should be small enough to concentrate the electric current, achieve high thermal energy, and render efficient tissue dissection. Conversely, to dissect highly vascularized soft tissue, the contact area should be large enough to decrease the electrical current concentration and obtain an efficient tissue coagulation effect. The size of the contact area is controlled by scope movement and device maneuvers. With regard to the sequence of the ESD procedure, a mucosal incision is performed in the cut mode because the mucosa contains the muscularis mucosae, which is a thin layer of tough, fibrous tissue. Submucosal dissection is performed in the cut mode if there are no vessels in the submucosa or fibrous tissue. Coagulation mode is used during submucosal dissection when vessels are present in the submucosa.
The settings of the cut mode (effect, duration, and interval) and coagulation mode (effect and wattage) are adjusted according to the type of ESD device used. The ESU settings for gastric ESD in our facility are shown in Tables 1-3.
ESD stages and electrosurgical settings (VIO 300D, Erbe) in our facility
| Knife | Marking | Mucosal incision | Submucosal dissection | Hemostasis |
| Needle-type knife: dual knife, Flush knife, etc. | · Forced COAG, E2, 25 W · Soft COAG, E4, 40 W | · EndoCut I, E2, D3, I3 | · Forced COAG, E2, 50 W · Swift COAG, E2, 50 W · EndoCut I, E2, D3, I3 | · Forced COAG, E2, 50 W (Minor bleeding) |
| Bended-tip needle knife type: hook knife | · Forced COAG, E2, 20 W · Soft COAG, E4, 40 W | · EndoCut I, E2, D3, I3 | · Forced COAG, E2, 50 W · Spray COAG, E2, 40 W | · Spray COAG, E2, 40-60 W (Minor bleeding) |
| Partially insulated-type knife: IT-knife 2, etc. | · EndoCut I, E2, D3, I3 | · Swift COAG, E3, 80 W · Forced COAG, E3, 50 W · EndoCut I, E2, D3, I3 | · Swift COAG, E3, 80 W · Forced COAG, E3 (Minor bleeding) | |
| Scissor type: clutch cutter, SB knife | · Forced COAG, E3, 20 W | · EndoCut Q, E1, D2, I1 (Coagulation before cutting: soft COAG, E4, 80W) | · EndoCut Q, E1, D2, I1 (Coagulation before cutting: soft COAG, E4, 80W) | · Soft COAG, E4, 80W |
| Hemostatic forceps: coagulasper, etc. | · Soft COAG, E5, 90W |
ESD stages and electrosurgical settings (VIO 3, Erbe) in our facility
| Knife | Marking | Mucosal incision | Submucosal dissection | Hemostasis |
| Needle-type knife: dual knife, flush knife, etc. | · Forced COAG, E 0.7 · Soft COAG, E4 | · EndoCUT I, E1, D3, I3 | · Forced COAG, E7.0 · EndoCUT I, E1, D3, I3 | · Forced COAG, E6.5 (Minor bleeding) |
| Bended-tip needle knife type: hook knife | · Forced COAG, Effect 4.5 · Soft COAG, E5 | · EndoCUT I, E1, D3, I3 · DRY CUT, E2.5 | · Spray COAG E4.7 · Forced COAG, Eeffect 7 | · Forced COAG, E6.5 (Minor bleeding) |
| Partially insulated-type knife: IT-knife 2, etc. | · EndoCUT I, E2, D3, I3 | · Swift COAG, Effect 6.0 · EndoCUT I, E2, D3, I3 (Submucosa without vessels) | · Swift COAG, Effect 6.0 (Minor bleeding) | |
| Hemostatic forceps: coagulasper, etc. | · Soft COAG, Effect 6.0 |
ESD stages and electrosurgical settings (ESG 300, Olympus) in our facility
| Knife | Marking | Mucosal incision | Submucosal dissection | Hemostasis |
| Needle-type knife: dual knife, Flush knife, etc. | · Forced COAG, Effect 2, 40 W · Soft COAG, Effect 3, 80 W | · Pulse CUT Fast, E2, 40-100 W | · Forced COAG, E3, 50 W · Pulse CUT Fast, E2, 40-100 W | · Forced COAG, E3, 50 W (Minor bleeding) |
| Partially insulated-type knife: hook knife | · Pulse CUT Fast, E3, 100 W | · Power COAG, E3, 80-120 W · Pulse CUT Fast, Effect 3, 100 W | · Power COAG, E3, 80-120 W (Minor bleeding) | |
| Hemostatic forceps: Coagulasper, etc. | · Soft COAG, Effect 3, 100 W |
ENDOSCOPIC PROCEDURE
Scope positioning and approach
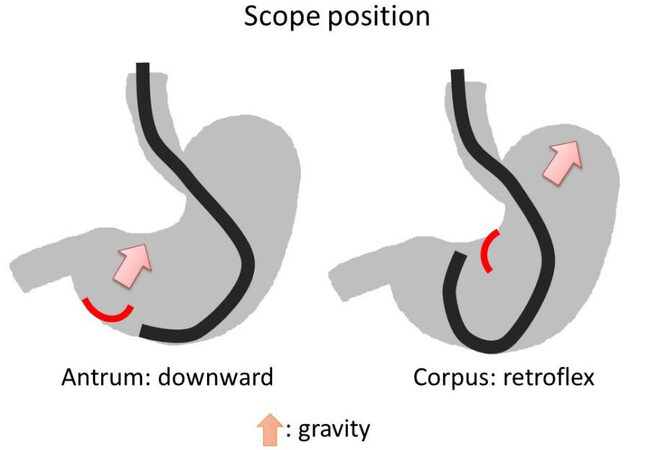
For a longitudinal location, a lesion in the antrum is generally approached from the oral side with the forward view, while a lesion in the corpus is approached from the anal side with the retroflex view because gravity in the stomach moves toward the fornix and lifts a dissected specimen up in these positions [Figure 4].
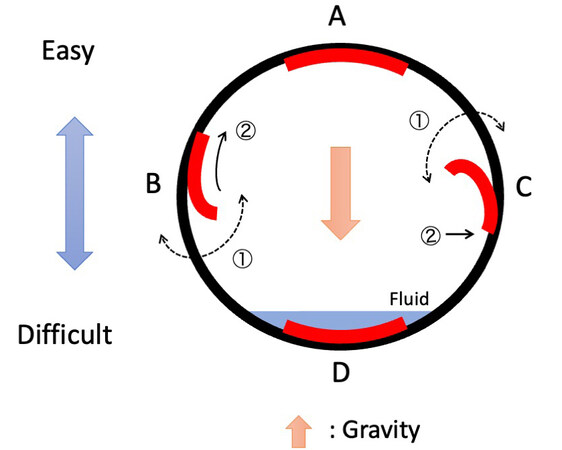
For a circumferential location, the difficulty of the ESD procedure can be assumed based on the direction of gravity [Figure 5]. The direction of gravity is distinguished by the presence of fluid. The dissection procedure for a lesion on the anti-gravity side is uncomplicated because the dissected specimen is spontaneously flipped up by gravity [Figure 5A]. For a lesion on the ipsilateral side of gravity, it is better to start a mucosal incision from the gravity side because the tension of mucosa from the anti-gravity side helps open the incised mucosal line [Figure 5B]. If the mucosal incision starts from the anti-gravity side, the lesion loses mucosal tension, shifts to the gravity side, and makes the mucosal incision on the gravity side difficult [Figure 5C]. A lesion on the gravity side presents the most difficult dissection position because spontaneous flapping of the dissected specimen by gravity cannot be achieved, and the fluid in the stomach interferes with the operation field. In this case, the traction device is useful. Changing the patient’s position to the right lateral is also worth considering as a possible solution [Figure 5D].
In the endoscopic view, with the use of scope torque and angulation, a lesion is principally positioned at the 6-7 o’clock location where a working channel exists because a device can be applied under good visualization of the mucosa in this position.
Figure 5. A. The lesion on the anti-gravity side is spontaneously flipped up by gravity. B. In the lesion on the ipsilateral side of gravity, mucosal incision is started from the gravity side (①) as the mucosal tension from the opposite side (②) helps open the incised mucosal line. C If the mucosal incision starts from the anti-gravity side (①), the mucosal incision on the gravity side becomes difficult. D. A lesion on the gravity side is the most difficult, and the traction device or changing the patient’s position can be a possible solution.
Because the anatomical shape of the stomach varies per individual, even in the same case, the appropriate positioning may change depending on the time and conditions. In particular, the shape of the stomach is affected by the amount of air inside, so it is better to pay attention to the extent of the lumen during the procedure and control the air volume by insufflation and deflation buttons. It is sometimes necessary to be flexible by switching the ESD knives or patient position during the gastric ESD procedure.
The appropriate position also depends on the type of ESD knife used. The operation field needs to be adjacent to the needle-type or scissor-type knives, while close positioning is not absolutely necessary for partially insulated knives. Therefore, in areas where close scope positioning is difficult, such as the gastric anterior wall or lesser curvature of the lower corpus, partially insulated knives are preferred.
Marking
The marking is performed 2-3 mm (approximately the width of the device sheath) outside using a needle-type knife with electrocautery under the careful image-enhanced endoscopic observation of the tumor boundary. Chromoendoscopy using indigo carmine and magnifying narrow-band imaging (NBI) share similar diagnostic accuracies for the delineation of early gastric cancer[8]. If there is a suspicious area adjacent to the lesion, it is included in the removal area; therefore, markings are made on an area diagnosed as definitely non-neoplastic outside the lesion. Then, a mucosal incision is performed outside the marking to completely remove the lesion and suspicious mucosa. In patients receiving H. pylori eradication therapy, a tumor will show surface differentiation[9], and the tumor surface may be partially covered with non-neoplastic epithelium[10]. Therefore, the tumor boundary is often unclear. It is preferable to add an extra margin for such lesions. In the case of a tumor with an unclear margin even under chromoendoscopic or magnifying NBI observation, a mapping biopsy is performed from the mucosa outside the suspicious tumor boundary. When biopsy results are negative, markings are made on the negative biopsy scars, and the mucosa outside the marking is incised. Markings can be applied subsequently around the lesion or initially in areas with clear demarcation and secondarily to areas between these markings. The marking interval is usually 2-3 mm or shorter, so the next marking is seen in the endoscopic view during mucosal incision. To orient the oral or anal side of the lesion in the resected specimen, extra markings can be placed on the oral or anal side as landmarks.
Submucosal injection
Submucosal fluid injection is performed just outside of the markings, where the mucosal incision is intended. Creating an adequate mucosal lift is important for safe ESD. The types of fluid used for submucosal injection include normal saline, glycerol, and sodium hyaluronate. Hypertonic solutions or viscous fluids are preferred because they elevate the mucosa more and maintain mucosal elevation longer than that by normal saline[11]. Submucosal injections should be administered into the appropriate layer. In general, injection into the shallow submucosa creates a high elevation for mucosal incision, whereas injection into the deep submucosa facilitates recognition of the deep submucosal plane for submucosal dissection. After puncturing the target mucosa, the needle tip is slightly maneuvered inside the submucosa to find the proper submucosal plane while the solution is injected. Once good elevation is achieved, the needle tip is planted in the same submucosal plane. Fine movement of the needle tip enables control of the blob creation direction. Puncture of the base of the previous elevation makes the subsequent injection smoother. As the injection solution is reabsorbed over time, injection and subsequent mucosal incision in large lesions are performed in multiple sessions instead of injecting the solution into the entire circumference in one session.
Mucosal incision
A partially insulated knife has an insulating tip; therefore, a pre-cut hole must be created at the most distal, surrounding mucosa with a needle knife. With a partially insulated knife, a mucosal incision is made from a pre-cut hole on the far side of the lesion to the near side and from the lateral part to the central part. In contrast, with a needle knife, a mucosal incision is made from the near side of the lesion to the far side and from the central part to the lateral part.
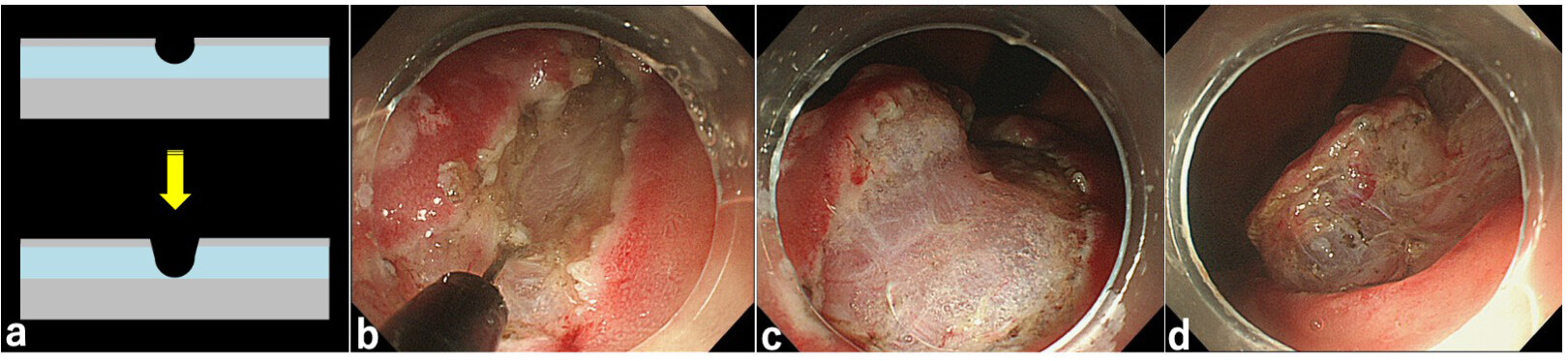
To reduce the incidence of bleeding during mucosal incision, a shallow mucosal incision is initially made with the endo-cut mode; then, the incision line is deepened with the coagulation mode (“trimming”), coagulating the submucosal vessels. This maneuver is particularly useful for lesions located in the corpus, where many thick vessels often exist in the submucosa. Trimming is often performed up to the plane between the submucosal vessels and the surface of the muscularis propria. The usefulness of trimming is as follows: (1) easy recognition of the initial submucosal plane for submucosal dissection; (2) efficient mucosal lift by submucosal injection to avoid leakage of the injected solution from the well-isolated specimen; and (3) easy completion of submucosal dissection at the lateral or distal part of the specimen [Figure 6].
Figure 6. (A) Schema of “trimming”. The trimming is performed until the surface of the muscularis propria. (B) Endoscopic image of trimming during endoscopic submucosal dissection (ESD). The muscularis propria behind the transparent submucosa is whitish. (C) After trimming and injection of solution into the submucosa, the initial submucosal plane for submucosal dissection is recognized easily. Isolation of the specimen from the normal mucosa avoids leakage of the injected solution and allows efficient mucosal lifting by submucosal injection. (D) After “trimming”, submucosal dissection at the lateral or distal part of the specimen can be easily performed. Note that the deep submucosa is less vascular and fibrous.
An entire circumferential incision is often made before submucosal dissection. However, a specimen is often shrunk in the center of a small lesion, making the creation of a flap for submucosal dissection difficult. In this case, a partial (approximately two-thirds) mucosal incision followed by subsequent submucosal dissection is performed so that the mucosal tension from the opposite side creates a flap, and the remaining mucosal incision and submucosal dissection are finalized.
Submucosal dissection
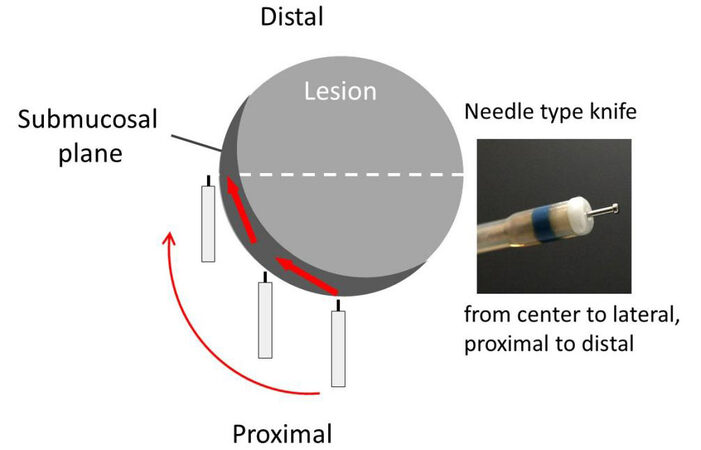
After injecting a solution into the deep submucosal plane, submucosal dissection is performed from the front side of the lesion. With a needle-type knife, submucosal dissection is initiated from the central part of the submucosa until the lateral edge [Figure 7].
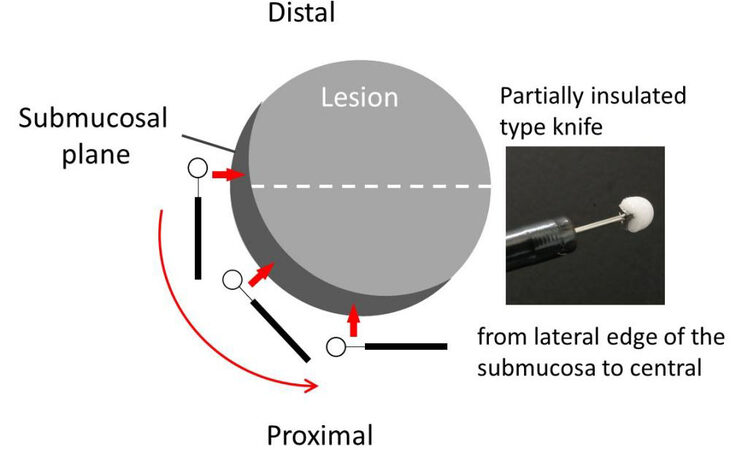
With the partially insulated knife, submucosal dissection is started from the tissue behind the lateral edge of the submucosa, until it reaches the central part [Figure 8].
Dissection of the lateral edge of the submucosa is important for the efficient creation of a mucosal flap. In gastric ESD, deep submucosal dissection just above the muscularis propria is important. The vessels in the gastric wall penetrate the muscularis propria and branch in the submucosa towards the mucosa. As the branches of the vessels in the shallow submucosa are surrounded by fibrous tissue, dissection of the superficial submucosa is difficult and may cause hemorrhagic oozing. In contrast, when dissecting the deep submucosa, although there is a possibility of encountering a thick vessel, there are fewer smaller blood vessels to encounter, and the submucosa between the vessels is less fibrous than that in the shallow submucosa [Figure 6D]. Moreover, during deep submucosal dissection, even when the vessels are severed, the bleeding vessel in the deep submucosa is easily recognized as a vascular stump on the surface of the muscularis propria, facilitating hemostasis.
Vessel coagulation
It is important to recognize the vessels in the submucosa during ESD for prophylactic coagulation. The vein appears reddish and is usually accompanied by an artery. The thin artery appears paler than the vein, and the thick artery appears whitish. The thin vessels are cauterized with an ESD knife using the coagulation mode. With the Dual knife, the thin vessels are cauterized by a small disk at the tip of the knife with the knife withdrawn. With the IT knife 2, it is cauterized by a triangle-shaped metal part. With the Flush Knife BT, the thin vessels are cauterized with the ball tip. IT Forced coagulation with a low high-frequency power setting (forced coagulation, Effect 1, 10 W) can be used for coagulation of thick vessels using an ESD knife[12]. The thick artery must be prophylactically coagulated with hemostatic forceps.
Oozing hemorrhage can be managed with coagulation using an ESD knife. If hemorrhagic oozing cannot be controlled by the application of knife coagulation multiple times, it is better to switch to forceps coagulation. In cases of arterial bleeding, hemostatic forceps are used for hemostasis. Identification of certain bleeding vessels or points is important to achieve successful hemostasis. The bleeding vessel or point is better to be bitted or compressed by the forceps to diminish active blood flow and to avoid the heat sink effect before application of electrocautery. Scope water-jet helps identification of the bleeding point. A new image-enhanced endoscopy mode, red dichromatic imaging (RDI), improves the visibility of bleeding points in acute upper gastrointestinal bleeding[13], but it did not reduce overall hemostasis time during gastric ESD in a multicenter prospective study[14]. Because we recognize the usefulness of the RDI mode for hemostasis of severe arterial bleeding in gastric ESD, it is always used in our practice.
Traction technique
When a lesion exists at the base of gravity and natural traction cannot be obtained, the traction method is effective in achieving a good view of the operative field during gastric ESD. Traction devices are generally divided into two types: external and internal. External traction includes the clip-with-line method[15], and internal traction includes S-O Clip[16] and multi-loop traction devices[17]. An RCT failed to show that the clip-with-line method reduces procedure time significantly in all cases compared to that by conventional ESD, although it was useful in lesions in the greater curvature and upper and middle parts of the stomach[18]. A single-center RCT comparing gastric ESD using an S-O clip and conventional ESD indicated a significant reduction in procedure time in the S-O clip group[19]. The usefulness of the traction method for lesions with severe submucosal fibrosis is suggested in some observational studies[20].
ADVERSE EVENTS AND MANAGEMENT
Delayed bleeding
Gastric ESD has a risk of delayed bleeding ranging from 3.6% to 6.9%[21-27]. A retrospective observational study indicated that prophylactic coagulation for all visible vessels at the base of post-ESD ulcers reduced the incidence of delayed bleeding and is commonly performed as a routine practice. Several RCTs and a meta-analysis indicated that proton pump inhibitor (PPI) administration significantly reduced the incidence of delayed bleeding compared with the administration of histamine-2 receptor antagonists (H2RA); thus, PPIs are recommended[28].
A meta-analysis identified several significant risk factors for delayed bleeding after gastric ESD including antithrombotic drug intake, chronic kidney diseases, resected specimen size > 30 mm, and use of an H2RA instead of a PPI[29]. The latest large-scale retrospective cohort study identified predictors for delayed bleeding after gastric ESD as warfarin, direct oral anticoagulant, chronic kidney disease with hemodialysis, P2Y12 receptor antagonist, aspirin, cilostazol, tumor size > 30 mm, lower-third in tumor location, presence of multiple tumors, and interruption of each kind of antithrombotic agents [Table 4][30]. The risk scoring system developed from the cohort can stratify bleeding risk as low risk (0-1 point, 2.8%), intermediate risk (2 points, 6.1%), high risk (3-4 points, 11.4%), and very high risk (≥ 5 points, 29.7%).
Predictors for delayed bleeding after ESD for EGC
| Variables | Adjusted odds ratio | 95%CI | P-value | β regression coefficient | Point | |
| CKD with hemodialysis | Yes | 4.33 | 2.71-6.91 | < 0.001 | 1.464 | 3 |
| Aspirin | Yes | 2.24 | 1.55-3.24 | < 0.001 | 0.807 | 2 |
| P2Y12RA | Yes | 3.13 | 1.91-5.12 | < 0.001 | 1.140 | 2 |
| Cilostazol | Yes | 2.04 | 1.09-3.80 | 0.025 | 0.712 | 1 |
| Warfarin | Yes | 8.74 | 4.92-15.54 | < 0.001 | 2.168 | 4 |
| DOAC | Yes | 8.16 | 4.74-14.04 | < 0.001 | 2.099 | 4 |
| Interruption of AT agents | Each kind of agents | 0.67 | 0.46-0.97 | 0.033 | -0.403 | -1 |
| The number of tumors | Multiple | 1.38 | 1.04-1.85 | 0.028 | 0.324 | 1 |
| Tumor size | > 30 mm | 1.72 | 1.28-2.31 | < 0.001 | 0.545 | 1 |
| Tumor location | Lower third | 1.68 | 1.35-2.10 | < 0.001 | 0.520 | 1 |
Several studies have indicated that the incidence of post-gastric ESD bleeding does not differ between patients treated with PPI and those treated with potassium-competitive acid blockers (P-CABs)[31-33]. A recent large-scale study using propensity score matching analysis suggested a significant reduction in post-gastric ESD bleeding in patients treated with P-CAB compared to those using PPI[34]. The use of antithrombotic agents is also a risk factor for delayed bleeding following gastric ESD. However, the Japanese Gastroenterological Endoscopy Society (JGES), European Society of Gastroenterological Endoscopy (ESGE), and American Society for Gastroenterological Endoscopy (ASGE) guidelines recommend performing high-bleeding-risk procedures without interruption of low-dose aspirin (LDA) therapy in patients who are at high risk for thromboembolic events[35-37]. Two meta-analyses of observational studies indicated similar delayed bleeding rates after gastric ESD between groups that continued and groups that interrupted their LDA therapy[38,39]. In terms of multiple antithrombotic therapies, the JGES, ESGE, and ASGE guidelines recommend that thienopyridine be withdrawn or replaced with aspirin monotherapy for high-bleeding-risk procedures. A meta-analysis indicated that the risk of delayed bleeding after gastric ESD in regular users of multiple antithrombotic drugs was significantly higher than that in non-users [odds ratio: 5.17 (95%CI: 3.13-8.54)][38].
In a recently reported multicenter retrospective study including 728 patients who received anticoagulants, delayed bleeding occurred in 14% of patients taking direct oral anticoagulants (DOACs), which was not considerably different from delayed bleeding rates in patients receiving warfarin (18%)[40]. The ESGE and ASGE guidelines recommend heparin bridging therapy during high-bleeding-risk endoscopic procedures, while the supplementary issue of the JGES guidelines for the management of patients taking anticoagulants suggests the possibility of continuation of warfarin instead of heparin bridging therapy[41]. Many studies have shown a high incidence of delayed bleeding after gastric ESD in patients receiving heparin bridging therapy (10.8%-61.5%)[42-49].
Some reports have described a new endoscopic method for the prevention of post-ESD bleeding in high-risk patients. Tissue shielding using polyglycolic acid (PGA) sheets and fibrin glue is one of the approaches to the prevention of post-ESD bleeding. Two prospective, non-randomized studies that enrolled patients taking antithrombotic agents showed that the delayed bleeding rate was significantly lower in the tissue shielding group than in the control group (6.7% vs. 22.0%, P = 0.04, and 5.8% vs. 20.8%, P = 0.041, respectively)[50,51]. Kikuchi et al. also reported favorable results for the PGA sheet shielding method in a single-arm study[52]. The PGA sheet delivery to post-ESD wounds through the working channel was technically demanding; however, Mori et al. developed a delivery station system and demonstrated the feasibility of the delivery devices[53]. A recent meta-analysis including four studies (212 lesions in the PGA sheet group and 208 in the control group) suggested that the post-ESD bleeding rate was significantly lower in the PGA sheet group than in the control group [4.9% vs. 13.7% (95%CI: 0.18-0.72)][54]. The usefulness of endoscopic closure of a post-ESD wound to prevent post-ESD bleeding in the stomach was also investigated. The latest multicenter RCT failed to demonstrate a significant effect of PGA sheet shielding on the prevention of post-ESD bleeding[55]; thus, further investigation is warranted.
Choi et al., in their observational study, indicated that endoclip closure of post-ESD wounds significantly reduced the delayed bleeding rate compared to that in the control group (3.3% vs. 13.3%, P = 0.04)[56]. However, a recent Japanese study revealed that clip and endoloop closure of post-gastric ESD ulcers did not reduce the incidence of post-ESD bleeding (n = 311, 11.5% vs. 11.9%, P = 0.89) in patients receiving antithrombotic therapy[57].
Perforations
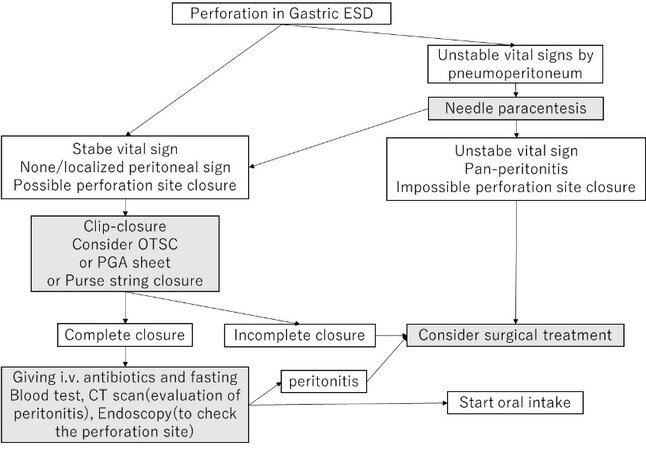
A systematic review identified a perforation rate of 3.02% (512/16,941), and 98.8% of patients (506/512) recovered without surgical intervention[58]. In general, intraoperative perforation during gastric ESD is initially managed with endoscopic clip closure. Needle paracentesis may be considered for unstable vital signs by pneumoperitoneum. If endoscopic clip closure is difficult, other methods (over-the-scope clips (OTSC), PGA sheet shielding, purse-string closure with endoclip-and-loop, etc.) are attempted. Unsuccessful endoscopic closure requires surgical intervention. Delayed perforation usually requires surgical intervention; however, endoscopic closure using OTSC or PGA sheets may be considered as a salvage treatment [Figure 9].
Figure 9. Management of perforation during gastric endoscopic submucosal dissection (ESD). OTSC: Over the scope clip; PGA: polyglycolic acid; CT: computed tomography.
Risk factors for intraoperative perforation during gastric ESD are shown in Table 5.
Risk factors for Intraoperative perforation of gastric ESD
| Risk factors |
| Location of the lesion (the corpus, the greater curvature, and remnant stomach) Large (> 20 mm) tumor size Submucosal tumor invasion or beyond Submucosal fibrosis Elevated morphology Long procedure time Piecemeal resection Prior workload of operators |
In terms of location, the upper third[59-65], middle third[65,66], greater curvature, and the remnant stomach[67] were reported as high-risk areas for intraprocedural perforation. Other risk factors include large tumor size[60,62], deep invasion[67,68], submucosal fibrosis[67-70], elevated morphology[67], and old age[68]. Long procedural time[63,68,71] and piecemeal resection[61] have also been reported to be associated with the incidence of perforation, which might be due to not only tumor-related factors but also endoscopist-related factors. Lim et al. suggested that operators’ workload, i.e., regular endoscopy work in an outpatient clinic prior to ESD, increased the risk of intraprocedural perforation[72].
A systematic review identified that the delayed perforation rate after gastric ESD was 0.19% (28/14,566), with 50% of patients (14/28) needing emergency surgery[58]. Several reports suggest that lesions in the gastric tube, those in the upper stomach, and excessive electrocautery during hemostasis are associated with delayed perforation[73-75].
Intraoperative perforation usually develops as a linear incision of the muscularis propria and is managed using simple clip closure. Air deflation is helpful in closing the incised perforation once a clip is applied. The omental-patch method may be indicated for large muscular defects, especially when they are located at the greater curvature of the gastric body[76]. Recently, new devices and methods such as OTSC[77], PGA sheet shielding[78,79], and closure using an endoclip-and-loop[73,80] have been reported in several case studies. All these methods could be used as a substitute when simple clip closure fails.
In cases of delayed perforation, the management is similar to that of intraoperative perforation. The difference may lie in the high comorbidity rate of pan-peritonitis in delayed perforation cases and friability of the tissues around the perforation hole, which may make simple clip closure difficult. Patients with delayed perforation who recovered with conservative management mostly developed adverse events (AE) before dietary intake and/or received endoscopic intervention within 24 h after onset[73,75].
Stenosis
A three-quarter circumferential resection is a risk factor for stenosis following gastric ESD. Prophylactic intralesional steroid injection and/or oral steroids have been reported, but their effectiveness for gastric stricture has not been fully verified. The main management method for gastric stenosis is endoscopic balloon dilation (EBD). Some observational studies indicated that one of the important risk factors for stenosis after gastric ESD in both the cardia and the pylorus was the size of the circumferential resection defect, which is often greater than three-fourths in extent[81-85].
The methods and efficacy of gastric stenosis prevention are controversial. One study reported that prophylactic EBD was effective in reducing the total number of EBD sessions[82]. A small study suggested combination treatment with oral and injectable steroids had preventative effects against gastric stenosis[86]. Three studies that investigated stenosis after pyloric ESD indicated the effectiveness of prophylactic EBD for the prevention of pyloric stenosis, but steroid injection was ineffective in one of four cases. Some reports described the use of a steroid (triamcinolone) solution for submucosal injection during ESD[87,88]. For the prevention of stenosis after both cardiac and pyloric ESD, administering oral steroids was more effective than the injection of steroids, and a combination of intralesional steroid injection and oral steroids did not seem to increase the preventive effect on stenosis[82,86,89].
Stenosis after gastric ESD is primarily treated by EBD. Two articles reported that there was no perforation related to EBD for cardiac stenosis[81,82], whereas perforation that developed during EBD for pyloric stenosis required emergency surgery[81-83,90]. There are two reports that describe the usefulness of mucosal incision of the stenotic ulcer scar and subsequent steroid injection[88,91].
Aspiration pneumonia
Apparent or silent pneumonia occurs in 0.62%-6.6% of patients undergoing gastric ESD[92,93]. Risk of pneumonia is increased in elderly patients (OR: 2.52, P < 0.00001)[94]. Other risk factors include a long
CONCLUSION
The preparation, techniques, and complications of gastric ESD are outlined herein. Mastery of ESD requires a deep understanding of not only the technique but also the electrosurgical unit, its peripherals, and probable procedural complications. The basics of the procedure are important, yet do not require anything special except expertise borne out of practice. Subsequently, the basic techniques for ESD incision and dissection should be mastered, and the appropriate knives, attachments, strategy, and traction device should be methodically selected by each operator.
DECLARATIONS
AcknowledgmentsWe thank Edanz Group (https://jp.edanz.com/english-editing-c) for editing a draft of this manuscript.
Authors’ contributionsDrafted the article: Nakamura T
Conception and design, critical revision of the article for important intellectual content, and final approval of the article: Uedo N
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc 2012;76:763-70.
2. Hasuike N, Ono H, Boku N, et al. A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer 2018;21:114-23.
3. Takizawa K, Ono H, Hasuike N, et al. Gastrointestinal Endoscopy Group (GIESG) and the Stomach Cancer Study Group (SCSG) of Japan Clinical Oncology Group. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer 2021;24:479-91.
4. Li X, Dong H, Zhang Y, Zhang G. CO2 insufflation versus air insufflation for endoscopic submucosal dissection: a meta-analysis of randomized controlled trials. PLoS One 2017;12:e0177909.
5. Baniya R, Upadhaya S, Khan J, et al. Carbon dioxide versus air insufflation in gastric endoscopic submucosal dissection: a systematic review and meta-analysis of randomized controlled trials. Clin Endosc 2017;50:464-72.
6. Takeuchi Y, Uedo N, Ishihara R, et al. Efficacy of an endo-knife with a water-jet function (Flushknife) for endoscopic submucosal dissection of superficial colorectal neoplasms. Am J Gastroenterol 2010;105:314-22.
7. Takeuchi Y, Shimokawa T, Ishihara R, et al. An electrosurgical endoknife with a water-jet function (flushknife) proves its merits in colorectal endoscopic submucosal dissection especially for the cases which should be removed en bloc. Gastroenterol Res Pract 2013;2013:530123.
8. Nagahama T, Yao K, Uedo N, et al. Delineation of the extent of early gastric cancer by magnifying narrow-band imaging and chromoendoscopy: a multicenter randomized controlled trial. Endoscopy 2018;50:566-76.
9. Kobayashi M, Hashimoto S, Nishikura K, et al. Magnifying narrow-band imaging of surface maturation in early differentiated-type gastric cancers after Helicobacter pylori eradication. J Gastroenterol 2013;48:1332-42.
10. Ito M, Tanaka S, Takata S, et al. Morphological changes in human gastric tumours after eradication therapy of Helicobacter pylori in a short-term follow-up. Aliment Pharmacol Ther 2005;21:559-66.
11. Huai ZY, Feng Xian W, Chang Jiang L, Xi Chen W. Submucosal injection solution for endoscopic resection in gastrointestinal tract: a traditional and network meta-analysis. Gastroenterol Res Pract 2015;2015:702768.
12. Ishida T, Toyonaga T, Ohara Y, et al. Efficacy of forced coagulation with low high-frequency power setting during endoscopic submucosal dissection. World J Gastroenterol 2017;23:5422-30.
13. Hirai Y, Fujimoto A, Matsutani N, et al. Evaluation of the visibility of bleeding points using red dichromatic imaging in endoscopic hemostasis for acute GI bleeding (with video). Gastrointest Endosc 2022;95:692-700.e3.
14. Fujimoto A, Saito Y, Abe S, et al. Clinical usefulness of red dichromatic imaging in hemostatic treatment during endoscopic submucosal dissection: first report from a multicenter, open-label, randomized controlled trial. Dig Endosc 2022;34:379-90.
15. Jeon WJ, You IY, Chae HB, Park SM, Youn SJ. A new technique for gastric endoscopic submucosal dissection: peroral traction-assisted endoscopic submucosal dissection. Gastrointest Endosc 2009;69:29-33.
16. Sakamoto N, Osada T, Shibuya T, et al. The facilitation of a new traction device (S-O clip) assisting endoscopic submucosal dissection for superficial colorectal neoplasms. Endoscopy 2008;40 Suppl 2:E94-5.
17. Matsui H, Tamai N, Futakuchi T, Kamba S, Dobashi A, Sumiyama K. Multi-loop traction device facilitates gastric endoscopic submucosal dissection: ex vivo pilot study and an inaugural clinical experience. BMC Gastroenterol 2022;22:10.
18. Yoshida M, Takizawa K, Suzuki S, et al. CONNECT-G Study Group. Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: a multicenter, randomized controlled trial (with video). Gastrointest Endosc 2018;87:1231-40.
19. Nagata M, Fujikawa T, Munakata H. Comparing a conventional and a spring-and-loop with clip traction method of endoscopic submucosal dissection for superficial gastric neoplasms: a randomized controlled trial (with videos). Gastrointest Endosc 2021;93:1097-109.
20. Abe S, Wu SYS, Ego M, et al. Efficacy of Current Traction Techniques for Endoscopic Submucosal Dissection. Gut Liver 2020;14:673-84.
21. Koh R, Hirasawa K, Yahara S, et al. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc 2013;78:476-83.
22. Lim JH, Kim SG, Kim JW, et al. Do antiplatelets increase the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms? Gastrointest Endosc 2012;75:719-27.
23. Miyahara K, Iwakiri R, Shimoda R, et al. Perforation and postoperative bleeding of endoscopic submucosal dissection in gastric tumors: analysis of 1190 lesions in low- and high-volume centers in Saga, Japan. Digestion 2012;86:273-80.
24. Toyokawa T, Inaba T, Omote S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol 2012;27:907-12.
25. Toyonaga T, Man-i M, East JE, et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc 2013;27:1000-8.
26. Kim TS, Kim B, Min BH, et al. Outcomes of endoscopic submucosal dissection for intestinal-type adenocarcinoma with anastomosing glands of the stomach. J Gastroenterol Hepatol 2020;35:50-5.
27. Nam HS, Choi CW, Kim SJ, et al. Risk factors for delayed bleeding by onset time after endoscopic submucosal dissection for gastric neoplasm. Sci Rep 2019;9:2674.
28. Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol 2007;102:1610-6.
29. Libânio D, Costa MN, Pimentel-Nunes P, Dinis-Ribeiro M. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc 2016;84:572-86.
30. Hatta W, Tsuji Y, Yoshio T, et al. Prediction model of bleeding after endoscopic submucosal dissection for early gastric cancer: BEST-J score. Gut 2021;70:476-84.
31. Toya Y, Endo M, Sugai K, et al. Protective effect of proton pump inhibitors and potassium competitive acid blockers against post-gastric endoscopic submucosal dissection bleeding: a single-center, propensity score-matched analysis. Scand J Gastroenterol 2021;56:199-204.
32. Hamada K, Uedo N, Tonai Y, et al. Efficacy of vonoprazan in prevention of bleeding from endoscopic submucosal dissection-induced gastric ulcers: a prospective randomized phase II study. J Gastroenterol 2019;54:122-30.
33. Kakushima N, Ono H, Takizawa K, et al. Incidence of delayed bleeding among patients continuing antithrombotics during gastric endoscopic submucosal dissection. Intern Med 2019;58:2759-66.
34. Abe H, Hatta W, Ogata Y, et al. Prevention of delayed bleeding with vonoprazan in upper gastrointestinal endoscopic treatment. J Gastroenterol 2021;56:640-50.
35. Fujimoto K, Fujishiro M, Kato M, et al. Japan Gastroenterological Endoscopy Society. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc 2014;26:1-14.
36. Veitch AM, Vanbiervliet G, Gershlick AH, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy 2016;48:385-402.
37. Acosta RD, Abraham NS, Chandrasekhara V, et al. ASGE Standards of Practice Committee. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc 2016;83:3-16.
38. Dong J, Wei K, Deng J, et al. Effects of antithrombotic therapy on bleeding after endoscopic submucosal dissection. Gastrointest Endosc 2017;86:807-16.
39. Jaruvongvanich V, Sempokuya T, Wijarnpreecha K, Ungprasert P. Continued versus interrupted aspirin use and bleeding risk after endoscopic submucosal dissection of gastric neoplasms: a meta-analysis. Ann Gastroenterol 2018;31:344-9.
40. Tomida H, Yoshio T, Igarashi K, et al. FIGHT-Japan Study Group. Influence of anticoagulants on the risk of delayed bleeding after gastric endoscopic submucosal dissection: a multicenter retrospective study. Gastric Cancer 2021;24:179-89.
41. Kato M, Uedo N, Hokimoto S, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment: 2017 appendix on anticoagulants including direct oral anticoagulants. Dig Endosc 2018;30:433-40.
42. Igarashi K, Takizawa K, Kakushima N, et al. Should antithrombotic therapy be stopped in patients undergoing gastric endoscopic submucosal dissection? Surg Endosc 2017;31:1746-53.
43. Yoshio T, Nishida T, Kawai N, et al. Gastric ESD under heparin replacement at high-risk patients of thromboembolism is technically feasible but has a high risk of delayed bleeding: Osaka university ESD study group. Gastroenterol Res Pract 2013;2013:365830.
44. Furuhata T, Kaise M, Hoteya S, et al. Postoperative bleeding after gastric endoscopic submucosal dissection in patients receiving antithrombotic therapy. Gastric Cancer 2017;20:207-14.
45. Yoshio T, Tomida H, Iwasaki R, et al. Effect of direct oral anticoagulants on the risk of delayed bleeding after gastric endoscopic submucosal dissection. Dig Endosc 2017;29:686-94.
46. Gotoda T, Hori K, Iwamuro M, et al. Evaluation of the bleeding risk with various antithrombotic therapies after gastric endoscopic submucosal dissection. Endosc Int Open 2017;5:E653-62.
47. Sanomura Y, Oka S, Tanaka S, et al. Taking warfarin with heparin replacement and direct oral anticoagulant is a risk factor for bleeding after endoscopic submucosal dissection for early gastric cancer. Digestion 2018;97:240-9.
48. Shindo Y, Matsumoto S, Miyatani H, Yoshida Y, Mashima H. Risk factors for postoperative bleeding after gastric endoscopic submucosal dissection in patients under antithrombotics. World J Gastrointest Endosc 2016;8:349-56.
49. Harada H, Suehiro S, Murakami D, et al. Continuous use of low-dose warfarin for gastric endoscopic submucosal dissection: a prospective study. Endosc Int Open 2017;5:E348-53.
50. Kawata N, Ono H, Takizawa K, et al. Efficacy of polyglycolic acid sheets and fibrin glue for prevention of bleeding after gastric endoscopic submucosal dissection in patients under continued antithrombotic agents. Gastric Cancer 2018;21:696-702.
51. Tsuji Y, Fujishiro M, Kodashima S, et al. Polyglycolic acid sheets and fibrin glue decrease the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms (with video). Gastrointest Endosc 2015;81:906-12.
52. Kikuchi D, Iizuka T, Nomura K, et al. Feasibility of autologous fibrin glue and polyglycolic acid sheets to prevent delayed bleeding after endoscopic submucosal dissection of gastric neoplasms in patients receiving antithrombotic therapy. Gastroenterol Res Pract 2018;2018:2174957.
53. Mori H, Guan Y, Kobara H, et al. Efficacy of innovative polyglycolic acid sheet device delivery station system: a randomized prospective study. Surg Endosc 2018;32:3076-86.
54. Li F, Xiong F, Xu ZL, Zhang DG, Yao J, Wang LS. Polyglycolic acid sheets decrease post-endoscopic submucosal dissection bleeding in early gastric cancer: a systematic review and meta-analysis. J Dig Dis 2020;21:437-44.
55. Kataoka Y, Tsuji Y, Hirasawa K, et al. Endoscopic tissue shielding to prevent bleeding after endoscopic submucosal dissection: a prospective multicenter randomized controlled trial. Endoscopy 2019;51:619-27.
56. Choi KD, Jung HY, Lee GH, et al. Application of metal hemoclips for closure of endoscopic mucosal resection-induced ulcers of the stomach to prevent delayed bleeding. Surg Endosc 2008;22:1882-6.
57. Ego M, Abe S, Nonaka S, Suzuki H, Yoshinaga S, Oda I, et al. Endoscopic closure utilizing endoloop and endoclips after gastric endoscopic submucosal dissection for patients on antithrombotic therapy. Dig Dis Sci 2021;66:2336-44.
58. Yamamoto Y, Kikuchi D, Nagami Y, et al. Management of adverse events related to endoscopic resection of upper gastrointestinal neoplasms: Review of the literature and recommendations from experts. Dig Endosc 2019;31 Suppl 1:4-20.
59. Onozato Y, Ishihara H, Iizuka H, et al. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy 2006;38:980-6.
60. Imagawa A, Okada H, Kawahara Y, et al. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy 2006;38:987-90.
61. Ohnita K, Isomoto H, Yamaguchi N, et al. Factors related to the curability of early gastric cancer with endoscopic submucosal dissection. Surg Endosc 2009;23:2713-9.
62. Ohta T, Ishihara R, Uedo N, et al. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest Endosc 2012;75:1159-65.
63. Yoon JY, Shim CN, Chung SH, et al. Impact of tumor location on clinical outcomes of gastric endoscopic submucosal dissection. World J Gastroenterol 2014;20:8631-7.
64. Yamaguchi N, Isomoto H, Fukuda E, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion 2009;80:173-81.
65. Kim HJ, Chung H, Jung DH, et al. Clinical outcomes of and management strategy for perforations associated with endoscopic submucosal dissection of an upper gastrointestinal epithelial neoplasm. Surg Endosc 2016;30:5059-67.
66. Akasaka T, Nishida T, Tsutsui S, et al. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc 2011;23:73-7.
67. Ojima T, Takifuji K, Nakamura M, et al. Complications of endoscopic submucosal dissection for gastric noninvasive neoplasia: an analysis of 647 lesions. Surg Laparosc Endosc Percutan Tech 2014;24:370-4.
68. Yoo JH, Shin SJ, Lee KM, et al. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc 2012;26:2456-64.
69. Kim JH, Nam HS, Choi CW, et al. Risk factors associated with difficult gastric endoscopic submucosal dissection: predicting difficult ESD. Surg Endosc 2017;31:1617-26.
70. Higashimaya M, Oka S, Tanaka S, et al. Outcome of endoscopic submucosal dissection for gastric neoplasm in relationship to endoscopic classification of submucosal fibrosis. Gastric Cancer 2013;16:404-10.
71. Mannen K, Tsunada S, Hara M, et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol 2010;45:30-6.
72. Lim SM, Park JC, Lee H, Shin SK, Lee SK, Lee YC. Impact of cumulative time on the clinical outcomes of endoscopic submucosal dissection in gastric neoplasm. Surg Endosc 2013;27:1397-403.
73. Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015;21:12635-43.
74. Hanaoka N, Uedo N, Ishihara R, et al. Clinical features and outcomes of delayed perforation after endoscopic submucosal dissection for early gastric cancer. Endoscopy 2010;42:1112-5.
75. Yamamoto Y, Nishisaki H, Sakai H, et al. Clinical Factors of Delayed Perforation after Endoscopic Submucosal Dissection for Gastric Neoplasms. Gastroenterol Res Pract 2017;2017:7404613.
76. Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc 2006;63:596-601.
77. Abe S, Minagawa T, Tanaka H, Oda I, Saito Y. Successful endoscopic closure using over-the-scope clip for delayed stomach perforation caused by nasogastric tube after endoscopic submucosal dissection. Endoscopy 2017;49:E56-7.
78. Ono H, Takizawa K, Kakushima N, Tanaka M, Kawata N. Application of polyglycolic acid sheets for delayed perforation after endoscopic submucosal dissection of early gastric cancer. Endoscopy 2015;47 Suppl 1 UCTN:E18-9.
79. Nagami Y, Shiba M, Arakawa T. Use of PGA sheets in the endoscopic closure of a perforation after endoscopic submucosal dissection for gastric-tube cancer. Am J Gastroenterol 2016;111:768.
80. Abe S, Oda I, Mori G, et al. Complete endoscopic closure of a large gastric defect with endoloop and endoclips after complex endoscopic submucosal dissection. Endoscopy 2015;47 Suppl 1 UCTN:E374-5.
81. Coda S, Oda I, Gotoda T, Yokoi C, Kikuchi T, Ono H. Risk factors for cardiac and pyloric stenosis after endoscopic submucosal dissection, and efficacy of endoscopic balloon dilation treatment. Endoscopy 2009;41:421-6.
82. Sumiyoshi T, Kondo H, Minagawa T, et al. Risk factors and management for gastric stenosis after endoscopic submucosal dissection for gastric epithelial neoplasm. Gastric Cancer 2017;20:690-8.
83. Lee JU, Park MS, Yun SH, et al. Risk factors and management for pyloric stenosis occurred after endoscopic submucosal dissection adjacent to pylorus. Medicine (Baltimore) 2016;95:e5633.
84. Kakushima N, Tanaka M, Sawai H, et al. Gastric obstruction after endoscopic submucosal dissection. United European Gastroenterol J 2013;1:184-90.
85. Iizuka H, Kakizaki S, Sohara N, et al. Stricture after endoscopic submucosal dissection for early gastric cancers and adenomas. Dig Endosc 2010;22:282-8.
86. Shoji H, Yamaguchi N, Isomoto H, et al. Oral prednisolone and triamcinolone injection for gastric stricture after endoscopic submucosal dissection. Ann Transl Med 2014;2:22.
87. Nishiyama N, Mori H, Kobara H, et al. Novel method to prevent gastric antral strictures after endoscopic submucosal dissection: using triamcinolone. World J Gastroenterol 2014;20:11910-5.
88. Mori H, Kobara H, Rafiq K, et al. Novel method for the management of stenosis after gastric endoscopic submucosal dissection: mucosal incision with steroid injection contralateral to the severely contracted scar. Dig Endosc 2015;27:622-6.
89. Kishida Y, Kakushima N, Takizawa K, et al. Effects of steroid use for stenosis prevention after wide endoscopic submucosal dissection for gastric neoplasm. Surg Endosc 2018;32:751-9.
90. Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008;67:979-83.
91. Mori H, Kobara H, Fujihara S, Nishiyama N, Rafiq K, Masaki T. Recanalization of severe gastric antral stricture after large endoscopic submucosal dissection: mucosal incision and local steroid injection. J. Gastrointestin. Liver Dis 2012;21:435-7.
92. Watari J, Tomita T, Toyoshima F, et al. The incidence of “silent” free air and aspiration pneumonia detected by CT after gastric endoscopic submucosal dissection. Gastrointest Endosc 2012;76:1116-23.
93. Gong EJ, Kim DH, Jung HY, et al. Pneumonia after endoscopic resection for gastric neoplasm. Dig Dis Sci 2014;59:2742-8.
94. Zhao J, Sun Z, Liang J, Guo S, Huang D. Endoscopic submucosal dissection for early gastric cancer in elderly vs. non-elderly patients: a systematic review and meta-analysis. Front Oncol 2021;11:718684.
95. Park CH, Kim H, Kang YA, et al. Risk factors and prognosis of pulmonary complications after endoscopic submucosal dissection for gastric neoplasia. Dig Dis Sci 2013;58:540-6.
96. Onozato Y, Kakizaki S, Ishihara H, Iizuka H, Sohara N, Okamura S, et al. Feasibility of endoscopic submucosal dissection for elderly patients with early gastric cancer and adenomas. Dig Endosc 2008;20:12-6.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Nakamura T, Uedo N. Endoscopic submucosal dissection for early gastric adenocarcinoma: basic procedures and management of complications. Mini-invasive Surg 2022;6:50. http://dx.doi.org/10.20517/2574-1225.2022.38
AMA Style
Nakamura T, Uedo N. Endoscopic submucosal dissection for early gastric adenocarcinoma: basic procedures and management of complications. Mini-invasive Surgery. 2022; 6: 50. http://dx.doi.org/10.20517/2574-1225.2022.38
Chicago/Turabian Style
Nakamura, Takahiko, Noriya Uedo. 2022. "Endoscopic submucosal dissection for early gastric adenocarcinoma: basic procedures and management of complications" Mini-invasive Surgery. 6: 50. http://dx.doi.org/10.20517/2574-1225.2022.38
ACS Style
Nakamura, T.; Uedo N. Endoscopic submucosal dissection for early gastric adenocarcinoma: basic procedures and management of complications. Mini-invasive. Surg. 2022, 6, 50. http://dx.doi.org/10.20517/2574-1225.2022.38
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 22 clicks
Cite This Article 22 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.