Which is the best algorithm for evaluating a patient's candidate to sleeve with suspected reflux or hiatal hernia: is manometry or reflux assessment always necessary
Abstract
Laparoscopic sleeve gastrectomy (SG) has reached wide popularity during the last 15 years, owing to limited morbidity and mortality rates, very successful weight loss results, and impact on comorbidities. However, the postoperative development or worsening of gastroesophageal reflux disease (GERD) is one of the most important drawbacks of this surgical procedure. To date, there is great heterogeneity concerning the definition of GERD, the indication for SG in patients with GERD, and the standardization of pre and postoperative diagnostic pathways. In patients with severe obesity, a strictly symptom-based diagnosis of GERD is unreliable. In fact, a high rate of silent GERD (s-GERD, asymptomatic patients despite objective evidence of GERD) has been reported. Moreover, patients with preoperative s-GERD have a significantly higher risk of experiencing GERD symptoms after SG. For these reasons, the reflux burden and the competence of the anti-reflux barrier should be carefully assessed during the preoperative work-up of patients undergoing SG. Ambulatory pH monitoring (APM) and high-resolution manometry (HRM) are useful diagnostic tools that could provide valuable evidence in the guidance of surgical strategy. In this review, we evaluate the current literature concerning the use of APM and HRM in the diagnostic pathway before SG, as well as their predictive value for the evolution of GERD in the postoperative course. Moreover, we propose a diagnostic algorithm for preoperative GERD assessment, which includes validated symptom questionnaires, upper gastrointestinal endoscopy, APM, and HRM.
Keywords
INTRODUCTION
Obesity is an independent risk factor for gastroesophageal reflux disease (GERD) and is associated with esophageal complications such as erosive esophagitis (EE), Barrett's esophagus (BE), and esophageal adenocarcinoma[1-3]. Multiple pathophysiological mechanisms predispose populations with obesity to GERD, including increased intra-abdominal pressure, decreased lower esophageal sphincter (LES) pressure, and higher frequency of transient LES relaxation (tLESR)[4]. In addition, a higher prevalence of hiatal hernia (HH) has been described[5].
Bariatric surgery has proven to be the most effective treatment for severe obesity (SO), yielding significant and sustained weight loss as well as the improvement of associated comorbidities and quality of life (QoL)[6,7]. Laparoscopic sleeve gastrectomy (SG) has become the most frequently performed procedure in the worldwide bariatric scenario[8-10] because of its simplicity, short operative times, and satisfying mid to long-term results with regard to weight loss and the improvement and resolution of comorbidities and QoL outcomes[8,9]. However, the postoperative development of de novo or worsening of existing GERD is currently the most troublesome and controversial issue of SG.
The pathophysiological relationship between SG and GERD is multifactorial and not completely understood. It has been claimed that SG entails the creation of a “low volume high-pressure system”, which may cause an imbalance between the intraluminal pressure of the gastric tubule and that of LES[11]. The former is physiologically increased because of the resection of the fundus, the most expandable portion of the stomach[12]. However, in case of technical errors (stenosis or twisting of the SG, resection of the antrum too radical), the increase of the intraluminal pressure may become pathological and could overcome that of LES, even if preserved. Alternatively, the surgical manipulation of the crural area during SG, with the resection of the sling fibers and the alteration of the His angle, may impair the competence of the esophagogastric junction (EGJ)[13-15]. Moreover, in cases of HH, the LES pressure can further decrease[16].
Data concerning the effect of SG on GERD are quite heterogeneous. In the last decade, several systematic reviews and meta-analyses have been published on this issue [Table 1]. The majority of these studies have reported the postoperative prevalence of GERD symptoms, the prevalence of EE, and the increased use of proton pump inhibitors (PPI) compared to preoperative rates. De novo GERD occurred in 12% to 69% of patients, whereas the incidence of BE after SG ranged from 8% to 11%. The rate of patients that were converted to Roux-en-Y gastric bypass (RYGB) because of severe reflux was up to 6.2%[17-24]. However, there is a lack of high-level evidence owing to the heterogeneity of the published studies concerning the definition of GERD, the selection criteria when offering surgery to those with or without GERD, and the standardization of the surgical technique. Furthermore, the methods to investigate and define reflux and its associated outcomes are variable, ranging from clinical notes to validated questionnaires, to upper gastrointestinal (UGI) endoscopy, objective reflux assessments (ambulatory pH monitoring, APM, and high-resolution manometry, HRM), or a combination of the above.
Summary of the current literature concerning the effect of Sleeve gastrectomy (SG) on Gastroesophageal reflux disease (GERD)
| Studies | Design | Post-operative gerd outcomes | ||||||
| GERD symptoms | de novoGERD | PPI use | EE | HH | BE | Conversion to RYGB | ||
| Chiu et al. (2011)[17] | SR | ■ Increased prevalence: 4 studies Decreased prevalence: 7 studies | n/a | n/a | n/a | n/a | n/a | n/a |
| Laffin et al. (2013)[18] | SR | ■ Increased prevalence: 8 studies (Increase range: 2.1%-34.9%) ■ Decreased prevalence: 5 studies (Decrease range: 2.8%-20%) | n/a | n/a | n/a | n/a | n/a | n/a |
| Oor et al. (2016)[19] | SR + MA | Slight not significant increase ■ Increased prevalence: 16 studies ■ Decreased prevalence: 12 studies | 20% | ■ Increased: 3 studies ■ Decreased: 1 study | New-onset: 6.3%-63% | n/a | n/a | n/a |
| Gu et al. (2019)[20] | SR + MA | Improvement/remission: 40.4% | 9.3% | ■ n/a | n/a | n/a | n/a | 6.2% |
| Yeung et al. (2019)[21] | SR + MA | Significant increase of GERD prevalence (19%) | 23% | ■ 38% | 30% | 41% | 8% | 4% |
| Qumseya et al. (2020)[22] | SR + MA | Significant increase of GERD prevalence (OR: 1.6-49) | 45% | ■ Before SG: 22%-24% ■ After SG: 73%-76% | Prevalence increase: 35%-86% | n/a | 11.4% | n/a |
| Balla et al. (2021)[23] | SR | Increased DMS and/or AET: 9 of 12 studies | 17.8%-69% | ■ n/a | n/a | n/a | n/a | n/a |
| *Chen et al. (2021)[24] | SR + MA | Remission: 68% | 12% | ■ n/a | n/a | 11% | n/a | n/a |
The present review aims to summarize the current evidence concerning the effectiveness of APM and HRM during the preoperative work-up of patients with SO seeking SG and to evaluate the predictive value of these tests for the evolution of GERD in the postoperative course. Moreover, the review includes a proposal of the GERD diagnostic algorithm for patients seeking SG and the means to implement the available data in clinical practice.
OBJECTIVE REFLUX ASSESSMENT MODALITIES
In clinical practice, GERD is empirically diagnosed and treated based on the assessment of symptoms by the clinician. However, in the case of treatment failure or diagnostic uncertainty, objective reflux testing can confirm or rule out the initial diagnosis. The 2018 Lyon Consensus provided recommendations for the use and interpretation of reflux testing techniques. UGI endoscopy and APM are considered first-line tests for GERD diagnosis, as they can provide conclusive evidence that confirms or excludes the presence of pathological reflux. In the case of inconclusive outcomes, further data concerning the morphology and the competence of the EGJ and the motility of the esophageal body can be inferred from HRM[25].
Monitoring of pH
APM can provide confirmatory evidence of GERD, in patients with normal endoscopy, atypical symptoms, and/or when contemplating anti-reflux surgery (ARS)[26]. The primary outcome metrics of APM are the acid exposure time (AET) and the number of reflux episodes (acidic, weakly acidic, or weakly alkaline). In 2018, the Lyon Consensus proposed that an AET of less than 4% could be considered definitively normal and above 6% could be considered definitively abnormal, with intermediate values between these limits being inconclusive. Over 80 reflux episodes per 24 hours are definitively abnormal, whereas below 40 is physiological, and intermediate values are inconclusive. In the case of inconclusive AET (4%-6%), reflux episodes > 80 or < 40 can confirm or rule out the diagnosis of GERD, respectively[25]. APM also provides the opportunity to assess the temporal relationship between the occurrence of reflux and the onset of symptoms[27]. The most valuable metrics are the symptom index (SI) and the symptom association probability (SAP). The former expresses the percentage of symptom episodes that are related to reflux, with a threshold of 50% to confirm this association. However, it does not take the total number of reflux episodes into account. On the other hand, the SAP expresses the likelihood that the patient’s symptoms are related to reflux and a value of ≥ 95% is considered positive. The combination of a positive SI and positive SAP provides the best evidence of a clinically relevant association between reflux episodes and symptoms and can support a GERD diagnosis when the other APM findings are inconclusive[28].
Finally, the DeMeester Score (DMS) is a composite score that measures acid exposure during APM. The mathematical calculation is based on points attributed to each standard deviation above the reference value for six parameters obtained from healthy individuals that serve as controls. The parameters that constitute the score are: (1) total number of episodes of reflux; (2) % total time esophageal pH < 4; (3) % upright time esophageal pH < 4; (4) supine time esophageal pH < 4; (5) number of reflux episodes ≥ 5 min; and (6) longest reflux episode (minutes)[29]. The sum of all parameters allows the diagnosis of pathologic reflux when the threshold value (14.72) is exceeded.
Esophageal manometry
Esophageal manometry assesses esophageal motility patterns by measuring the amplitude of contractile events in the esophagus and its sphincters in relation to time. HRM represents an evolution from conventional manometry. The main difference between the two manometric systems is the number of pressure sensors on the esophageal catheters. In contrast to conventional manometry, where three to eight sensors are spaced at 3 to 5 cm intervals, HRM sensors are typically spaced 1 cm apart along the length of the manometric assembly[30].
In GERD patients, esophageal manometry is usually performed to rule out major motor disorders (particularly achalasia), assess esophageal peristaltic performance before ARS, and evaluate post-fundoplication dysphagia. Emerging indications are represented by the assessment of morphology and integrity of the EGJ, the measurement of hiatus hernia size, and the assessment of esophageal peristaltic performance before bariatric procedures[31]. The impairment of the EGJ as an anti-reflux barrier is the most important abnormality in GERD. Thus, the quantification of EGJ competence represents a significant biomarker in GERD evaluation. The Lyon Consensus proposed the adoption of two HRM parameters to evaluate EGJ competence adequately, one expressing its anatomical morphology and the second summarizing its contractile strength[25].
The EGJ morphology is defined by the spatial relationship between the LES and crural diaphragm (CD) and has been characterized into three subtypes according to the Chicago Classification of esophageal motility disorders, v3.0: Type I, no axial separation between the LES and the CD; Type II, minimal separation
The second parameter is the EGJ-contractile integral (EGJ-CI), which provides an overall measurement of EGJ contractility[34]. Unlike other manometric markers that are commonly used to evaluate the anti-reflux barrier function (integrated relaxation pressure, expiratory, and inspiratory EGJ pressures), EGJ-CI allows the contractility of the EGJ to be evaluated at rest, which is fundamental in determining its competence[35]. It has been shown that both the disruption of the EGJ (type II and III) and a defective EJG (EGJ-CI values below 39 mmHg·cm) are significantly associated with increased acid exposure. Thus, the evaluation of these two metrics can be useful in predicting abnormal APM outcomes[36,37].
Esophageal peristalsis can be characterized by the distal contractile integral (DCI), which is an assessment of the vigor of esophageal smooth muscle contraction. Esophageal motility is considered intact when the DCI value is above 450 mmHg·cm·s. On the contrary, a DCI value < 450 mm Hg·cm·s indicates weak peristalsis[25]. The Chicago Classification of esophageal motility disorders (v4.0), defines ineffective esophageal motility (IEM) as ≥ 70% of test swallows with DCI < 450 mm Hg·cm·s[38]. High proportions of ineffective contractions increase the likelihood of abnormal esophageal acid exposure and reflux symptoms, whereas the greatest reflux burden is seen with absent contractility (100% of test swallows with DCI < 100 mm Hg·cm·s)[39,40].
The evidence about the manometric and pH monitoring changes after SG is heterogeneous, likewise is that from the articles evaluating the impact of SG on GERD symptoms. However, the majority of studies have reported the postoperative worsening of these functional parameters[23]. This variability might depend on the use of different techniques (e.g., conventional manometry vs. HRM), the parameters analyzed, and the variable length of postoperative follow-up.
In studies involving the use of conventional manometry, a significant impairment of LES tone and esophageal body motility is demonstrated. Braghetto et al. showed a significant decrease in both the resting pressure and the length of the LES in 20 patients at six months after SG, with an 85% incidence of de novo hypotensive LES. The authors hypothesized that the partial resection of the sling fibers during surgery might be the cause of a hypotensive LES and, consequently, the development of de novo GERD[14]. Similar results were observed by Valezi et al., who reported a significant decrease of LES pressure in 73 patients one year after SG, as compared to preoperative values. Furthermore, they found a significant reduction in the amplitude of the esophageal peristaltic waves and the percentage of patients with normal peristalsis[41]. In contrast, Rebecchi et al. did not find any difference in LES resting pressure and distal esophageal wave amplitude in 65 patients two years after SG[42].
However, in studies involving the use of HRM, it has been observed that SG affected esophageal peristalsis rather than the competence of the LES. Coupaye et al. reported a significant reduction of both the percentage of normal esophageal contraction and DCI 1 year after surgery in a series of 47 patients, whereas the incidence of IEM significantly increased compared to the baseline value (from 20% to 50%; P = 0.048). In contrast, no significant changes in LES resting pressure and EGJ-CI were observed[43]. Similar results were reported by Del Genio et al. in a series of 25 patients showing unchanged LES function after SG. The incidence of IEM significantly increased from 10% at baseline to 46% after surgery
Concerning pH monitoring, most of the published studies reported a worsening of the reflux burden after SG. Coupaye et al. observed a significant increase in total AET and DMS in the entire cohort. However, when patients were stratified using baseline APM, reflux parameters worsened significantly only in the group of patients without baseline GERD, whereas no significant changes were observed in patients with baseline GERD[43]. Similarly, Tolone et al. observed a significant increase in total AET and the total number of reflux episodes one year after SG in a cohort of 26 patients without baseline pathological pH monitoring[45]. In contrast, Yormaz et al. reported a significant postoperative improvement of the reflux burden in a cohort of 152 patients assessed by APM at 6, 12, and 24 months after SG. In the entire cohort, DMS and total AET decreased significantly at 6 and 12 months, whereas no significant changes were observed at 24 months after surgery. Interestingly, the authors reported that weight loss outcomes and APM parameters were significantly better in the group of patients who underwent a gastric resection at 2 cm from the pylorus, compared to the group of patients who underwent gastric resection at 6 cm, claiming that the antral resection may have a possible effect on postoperative GERD outcomes[46].
GERD ASSESSMENT IN PATIENTS WITH SO - THE ISSUE OF SILENT GERD (s-GERD)
In patients with SO, a strictly symptom-based diagnosis of GERD is unreliable. In fact, a high rate of s-GERD (asymptomatic patients despite objective evidence of GERD) has been reported[47,48]. Carabotti et al. investigated 142 patients with SO before bariatric surgery with a validated Rome III symptomatic questionnaire and UGI endoscopy. They found that 59.7% of patients with abnormal endoscopic findings were asymptomatic. Moreover, they estimated that 87.5% of EE would have been missed by only performing preoperative endoscopy in patients with significant symptoms[49].
Ortiz et al. reported that only 22 out of 75 patients with objective data of abnormal gastroesophageal reflux (esophagitis and/or positive APM) satisfied the symptomatic criteria of GERD, while the remaining 53 patients with EE and/or abnormal APM outcomes were asymptomatic[50]. Heimgartner et al. assessed the prevalence of reflux symptoms and abnormal APM findings in 100 patients seeking bariatric surgery. They reported that abnormal APM was noted in 69% of symptomatic and 50% of asymptomatic patients, whereas no differences were found between symptomatic and asymptomatic patients with regard to AET values. Thus, the authors concluded that endoscopy and reflux monitoring should be part of the preoperative work-up before bariatric surgery, especially before restrictive procedures[51].
The pathogenesis of s-GERD is uncertain. It has been hypothesized as a reduced esophageal sensitivity in patients with SO compared to patients without obesity. Ortiz et al. compared 30 patients with SO to 28 non-obese controls and the former were found to be significantly less symptomatic (14% vs. 96%) and to have decreased esophageal sensitivity (57% vs. 14%) when acid was instilled in the esophagus during APM[48]. Dysfunction of the autonomic nervous system characterized by the progressive decrease in sympathetic and parasympathetic activity has been observed in patients with SO, which could account for an altered esophageal sensitivity in these patients[52].
The high incidence of s-GERD in patients with SO undergoing SG significantly increases the risk of experiencing GERD symptoms in their postoperative course. Borbély et al. reported a 25% occurrence of
Abnormal manometric parameters are also frequent in patients with SO undergoing SG. Kristo et al. performed UGI endoscopy, APM, and HRM in 177 asymptomatic patients undergoing bariatric surgery. They reported a structural defective LES in 36.2% of patients and a motility disorder in 35.6% of patients. Moreover, the authors calculated that, according to the Lyon Consensus criteria, 31.1% of the study cohort had a conclusive GERD diagnosis, whereas another 44.1% had borderline GERD. This resulted in 75.2% of asymptomatic individuals with SO being affected by some form of GERD before bariatric surgery[54].
To date, there is no evidence supporting the use of HRM before bariatric surgery as a predictive factor of postoperative GERD development or worsening. Soliman et al. reported that no manometric outcomes were correlated with postoperative GERD symptoms[53]. Similarly, other studies have failed to demonstrate a correlation between preoperative manometric findings and the occurrence of postoperative GERD symptoms[44,55,56].
One possible explanation lies with the profound anatomical modifications entailed by the SG, which changes the stomach from a low to a high-pressure system. The increase of the intraluminal pressure generates a gastroesophageal pressure gradient that could overcome EGJ competence, even if preserved. Consequently, the preoperative evidence of normal EGJ function may not predict the beneficial impact of SG on GERD reliably[56]. Moreover, it has been shown that the surgical manipulation of the crural area during SG may impair EGJ competence[14]. Quero et al. prospectively investigated 23 patients with APM, HRM, and magnetic resonance imaging (MRI) before and more than six months after SG. APM and HRM showed a significant postoperative worsening of the reflux burden and EGJ competence, respectively[15]. On the other hand, the assessment of the EGJ and gastric morphology by MRI pointed out a significant increase of the His angle’s degree after SG. The authors reported that these changes in the local anatomy were significantly correlated either with an increase in esophageal reflux exposure or with the impairment of EGJ competence. Moreover, the patients with the largest reduction in gastric volume after SG (≥ 80%) had a significant increase in postoperative supine non-acid refluxes and incidence of GERD symptoms[15].
GERD DIAGNOSTIC ALGORITHM IN PATIENTS WITH SO UNDERGOING SG
Considering the huge number of SG performed, the possible detrimental implications on GERD, and the high incidence of s-GERD in patients with SO, the preoperative work-up should include a proper evaluation of both the reflux burden and the competence of the anti-reflux barrier. The above-mentioned evidence suggests that APM and HRM may represent useful diagnostic tools, as they provide relevant information that could guide the surgeon’s decision. Obviously, submitting all the patients awaiting SG to both APM and HRM or, likewise, the preoperative work-up of patients undergoing ARS, is unrealistic.
In fact, the number of bariatric procedures performed yearly is considerably higher than that of fundoplication interventions. In the US, the number of elective anti-reflux procedures performed yearly between 2005 and 2010 ranged between 15,819 and 18,780, with a decline of about 50% since the peak of 32,980 reached in 2000[57,58]. On the other hand, in 2016, 127,318 SGs were performed in the US, according to the survey of the International Federation for the Surgery of Obesity and Metabolic Disorders[10]. Moreover, the survey reported that the number of SGs performed worldwide in 2016 was 340,550 (accounting for 53.6% of all bariatric procedures), a steep increase compared to previous surveys[10,59].
Therefore, preoperative work-up before SG should be tailored on the basis of a diagnostic GERD algorithm that includes validated symptom questionnaires, UGI endoscopy, APM, and HRM. This might avoid unnecessary, costly, and time-consuming exams and would reduce the rate of misdiagnosed GERD.
This issue was addressed in the First International Consensus Conference on gastroesophageal reflux and SG, held in 2019, involving 50 international bariatric experts from 25 countries[11]. Of the consensus panel members, 91.9% claimed that UGI endoscopy should be mandatory during the work-up of patients before SG, to rule out pathological findings that may change surgical planning. In particular, the experts agreed that the appearance of a severe EE (LA classification D) or BE with intestinal metaplasia, represented an absolute contraindication to performing SG and that RYGB was the best surgical option in patients with these endoscopic findings. There was no consensus among the experts to consider the mandatory use of UGI series, APM, and HRM in the preoperative work-up, even in symptomatic candidates with GERD symptoms, any degree of EE, or BE[11].
To date, only one study has dealt with the implementation of a GERD diagnostic algorithm before SG. In this study, the initial preoperative evaluation consisted of UGI endoscopy and the application of a GERD Health-Related QoL (GERD-HRQL) questionnaire. Patients with EE and/or a GERD-HRQL score > 28, underwent APM. If the resulting DMS was > 14.7, LSG with concomitant posterior HH repair (HHR) was performed.
It was reported that 84.2% of the patients who underwent the APM had a DMS > 14.7 and were submitted to HHR. APM was repeated 12 months after the surgical procedure, showing a significant improvement in esophageal acid exposure. Moreover, only 3.3% of patients had a DMS > 14.7. The authors claimed that the application of this algorithm allowed a careful selection of high-risk patients for GERD, avoiding unnecessary dissection of the diaphragmatic crus[60].
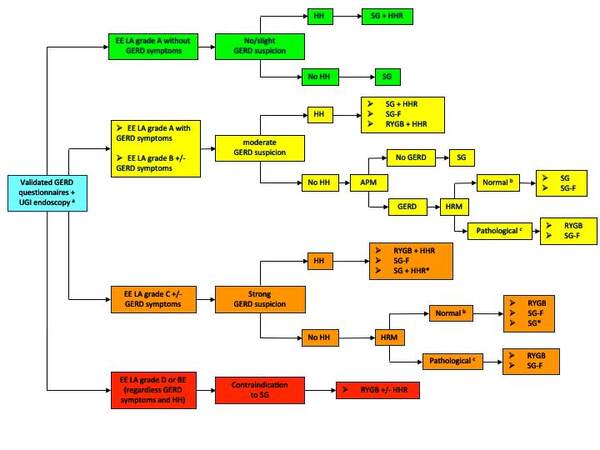
Hereafter, a GERD diagnostic pathway in patients undergoing SG is proposed [Figure 1]. Different degrees of suspected GERD have been defined using the validated GERD questionnaires and UGI endoscopy, which are considered first-line tests to be performed in all patients awaiting SG. In patients with severe EE (LA grade D) and BE, SG is considered contraindicated[11], whereas, in patients with a strong suspicion of GERD (EE grade C), SG with or without HHR should be performed only in selected cases (specific patient’s request, contraindication to RYGB). The use of APM is suggested in patients with moderate suspicion of GERD without endoscopic evidence of HH. On the contrary, in patients with a strong suspicion of GERD, APM is considered redundant as the presence of high-grade EE (LA grade C) is predictive of pathological reflux.
Figure 1. Preoperative diagnostic algorithm in patients undergoing SG. APM: ambulatory pH monitoring; EE: erosive esophagitis; GERD: gastroesophageal reflux disease; HH: hiatal hernia; HHR: hiatal hernia repair; HRM: high-resolution manometry; LA: Los Angeles classification; SG: sleeve gastrectomy; SG-F: sleeve gastrectomy combined with fundoplication; RYGB: Roux-en-Y gastric bypass; UGI: upper gastrointestinal. aUpper gastrointestinal series is optional. bEffective esophagogastric junction (EGJ); no Lower esophageal sphincter-crural diaphragm (LES-CD) disruption (type I): according to the Chicago Classification of esophageal motility disorders, v3.0.
The use of HRM to assess the competence of the anti-reflux barrier is suggested in pathological APM or patients with a strong suspicion of GERD without endoscopic evidence of HH. When the HRM shows anatomical separation between LES and CD (type II or III) and/or the presence of a defective EGJ, the exploration of the crural area and, possibly, the HHR is indicated. In patients with confirmed GERD diagnosis and/or manometric evidence of esophageal motility disorder, the surgical indications could be switched to RYGB. During the First International Consensus Conference on gastroesophageal reflux disease, 77.3% of the consensus panel would have considered SG combined with fundoplication (SG-F) as a surgical option in patients with SO and GERD symptoms, whereas consensus was not achieved for SG-F in patients with higher degrees of GERD such as severe esophagitis (LA grade D) or BE[11]. Therefore, SG-F has been included in the algorithm as an alternative to SG.
The present algorithm has some limitations. First, the diagnosis of symptom-based GERD is less accurate than physiology-based GERD diagnosis (defined by objective reflux tests). However, considering the huge number of patients undergoing SG, the use of validated GERD questionnaires (rather than informal reporting of reflux symptoms) in association with routine UGI endoscopy could be valuable and cost-effective as a primary step in the diagnostic pathway for GERD before SG. Moreover, the algorithm represents an attempt to translate the above-mentioned evidence from literature into daily clinical practice. Nevertheless, it has not been tested in a clinical trial and no conclusions concerning its effectiveness can be drawn.
CONCLUSIONS
The postoperative development of de novo or worsening of existing GERD is currently the most troublesome and controversial issue of SG. The creation of a high-pressure intragastric system and the manipulation of the crural area are intrinsic refluxogenic factors of this surgical procedure, and their detrimental effects on the anti-reflux barrier can be only partially lightened by following specific technical rules (avoiding stenosis or twisting of the gastric tubule, selective dissection of the crural area, and HHR). Moreover, these pathophysiological changes can further worsen pre-existing GERD. For this reason, the careful assessment of the reflux burden and the EGJ morphology before SG is essential, particularly in those patients with s-GERD. APM and HRM are useful diagnostic tools that can provide valuable evidence to guide the surgical strategy. However, the cost-effectiveness ratio of their implementation in the preoperative diagnostic algorithm should be assessed in clinical trials involving a larger number of patients.
DECLARATIONS
Authors’ contributionMade substantial contributions to conception and design of the study: Soricelli E, Facchiano E, Lucchese M
Performed review of the literature: Soricelli E, Casella G, Genco A
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol 2005;100:1243-50.
2. El-Serag HB. Obesity and disease of the esophagus and colon. Gastroenterol Clin North Am 2005;34:63-82.
3. El-Serag H. The association between obesity and GERD: a review of the epidemiological evidence. Dig Dis Sci 2008;53:2307-12.
4. El-Serag HB, Ergun GA, Pandolfino J, Fitzgerald S, Tran T, Kramer JR. Obesity increases oesophageal acid exposure. Gut 2007;56:749-55.
5. Csendes A, Burgos AM, Smok G, Beltran M. Endoscopic and histologic findings of the foregut in 426 patients with morbid obesity. Obes Surg 2007;17:28-34.
6. O’Brien PE, Hindle A, Brennan L, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg 2019;29:3-14.
7. Hachem A, Brennan L. Quality of life outcomes of bariatric surgery: a systematic review. Obes Surg 2016;26:395-409.
8. English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American society for metabolic and bariatric surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis 2018;14:259-63.
9. Welbourn R, Hollyman M, Kinsman R, et al. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg 2019;29:782-95.
10. Angrisani L, Santonicola A, Iovino P, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg 2018;28:3783-94.
11. Assalia A, Gagner M, Nedelcu M, Ramos AC, Nocca D. Gastroesophageal reflux and laparoscopic sleeve gastrectomy: results of the first international consensus conference. Obes Surg 2020;30:3695-705.
12. Yehoshua RT, Eidelman LA, Stein M, et al. Laparoscopic sleeve gastrectomy-volume and pressure assessment. Obes Surg 2008;18:1083-8.
13. Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis 2017;13:568-74.
14. Braghetto I, Lanzarini E, Korn O, Valladares H, Molina JC, Henriquez A. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes Surg 2010;20:357-62.
15. Quero G, Fiorillo C, Dallemagne B, et al. The causes of gastroesophageal reflux after laparoscopic sleeve gastrectomy: quantitative assessment of the structure and function of the esophagogastric junction by magnetic resonance imaging and high-resolution manometry. Obes Surg 2020;30:2108-17.
16. Tolone S, Savarino E, de Bortoli N, et al. Esophagogastric junction morphology assessment by high resolution manometry in obese patients candidate to bariatric surgery. Int J Surg 2016;28 Suppl 1:S109-13.
17. Chiu S, Birch DW, Shi X, Sharma AM, Karmali S. Effect of sleeve gastrectomy on gastroesophageal reflux disease: a systematic review. Surg Obes Relat Dis 2011;7:510-5.
18. Laffin M, Chau J, Gill RS, Birch DW, Karmali S. Sleeve gastrectomy and gastroesophageal reflux disease. J Obes 2013;2013:741097.
19. Oor JE, Roks DJ, Ünlü Ç, Hazebroek EJ. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Surg 2016;211:250-67.
20. Gu L, Chen B, Du N, et al. Relationship between bariatric surgery and gastroesophageal reflux disease: a systematic review and meta-analysis. Obes Surg 2019;29:4105-13.
21. Yeung KTD, Penney N, Ashrafian L, Darzi A, Ashrafian H. Does sleeve gastrectomy expose the distal esophagus to severe reflux? Ann Surg 2020;271:257-65.
22. Qumseya BJ, Qumsiyeh Y, Ponniah SA, et al. Barrett’s esophagus after sleeve gastrectomy: a systematic review and meta-analysis. Gastrointest Endosc 2021;93:343-352.e2.
23. Balla A, Meoli F, Palmieri L, et al. Manometric and pH-monitoring changes after laparoscopic sleeve gastrectomy: a systematic review. Langenbecks Arch Surg 2021;406:2591-609.
24. Chen W, Feng J, Wang C, et al. ; Chinese Obesity and Metabolic Surgery Collaborative. Effect of concomitant laparoscopic sleeve gastrectomy and hiatal hernia repair on gastroesophageal reflux disease in patients with obesity: a systematic review and meta-analysis. Obes Surg 2021;31:3905-18.
25. Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon consensus. Gut 2018;67:1351-62.
26. Roman S, Gyawali CP, Savarino E, et al. ; GERD consensus group. Ambulatory reflux monitoring for diagnosis of gastro-esophageal reflux disease: update of the porto consensus and recommendations from an international consensus group. Neurogastroenterol Motil 2017;29:1-15.
27. Bredenoord AJ, Weusten BL, Smout AJ. Symptom association analysis in ambulatory gastro-oesophageal reflux monitoring. Gut 2005;54:1810-7.
28. Kushnir VM, Sathyamurthy A, Drapekin J, Gaddam S, Sayuk GS, Gyawali CP. Assessment of concordance of symptom reflux association tests in ambulatory pH monitoring. Aliment Pharmacol Ther 2012;35:1080-7.
29. Johnson LF, Demeester TR. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am J Gastroenterol 1974;62:325-32.
30. Yadlapati R. High-resolution esophageal manometry: interpretation in clinical practice. Curr Opin Gastroenterol 2017;33:301-9.
31. Savarino E, Bredenoord AJ, Fox M, et al. Erratum: advances in the physiological assessment and diagnosis of GERD. Nat Rev Gastroenterol Hepatol 2018;15:323.
32. Kahrilas PJ, Bredenoord AJ, Fox M, et al. ; International High Resolution Manometry Working Group. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74.
33. Weijenborg PW, van Hoeij FB, Smout AJ, Bredenoord AJ. Accuracy of hiatal hernia detection with esophageal high-resolution manometry. Neurogastroenterol Motil 2015;27:293-9.
34. Nicodème F, Pipa-Muniz M, Khanna K, Kahrilas PJ, Pandolfino JE. Quantifying esophagogastric junction contractility with a novel HRM topographic metric, the EGJ-Contractile Integral: normative values and preliminary evaluation in PPI non-responders. Neurogastroenterol Motil 2014;26:353-60.
35. Xie C, Wang J, Li Y, et al. Esophagogastric junction contractility integral reflect the anti-reflux barrier dysfunction in patients with gastroesophageal reflux disease. J Neurogastroenterol Motil 2017;23:27-33.
36. Ham H, Cho YK, Lee HH, et al. Esophagogastric junction contractile integral and morphology: two high-resolution manometry metrics of the anti-reflux barrier. J Gastroenterol Hepatol 2017;32:1443-9.
37. Tolone S, De Bortoli N, Marabotto E, et al. Esophagogastric junction contractility for clinical assessment in patients with GERD: a real added value? Neurogastroenterol Motil 2015;27:1423-31.
38. Yadlapati R, Pandolfino JE, Fox MR, Bredenoord AJ, Kahrilas PJ. What is new in Chicago classification version 4.0? Neurogastroenterol Motil 2021;33:e14053.
39. Blonski W, Vela M, Safder A, Hila A, Castell DO. Revised criterion for diagnosis of ineffective esophageal motility is associated with more frequent dysphagia and greater bolus transit abnormalities. Am J Gastroenterol 2008;103:699-704.
40. Reddy CA, Patel A, Gyawali CP. Impact of symptom burden and health-related quality of life (HRQOL) on esophageal motor diagnoses. Neurogastroenterol Motil 2017;29:e12970.
41. Valezi AC, Herbella FA, Mali-Junior J, Menezes MA, Liberatti M, Sato RO. Preoperative manometry for the selection of obese people candidate to sleeve gastrectomy. Arq Bras Cir Dig 2017;30:222-4.
42. Rebecchi F, Allaix ME, Giaccone C, Ugliono E, Scozzari G, Morino M. Gastroesophageal reflux disease and laparoscopic sleeve gastrectomy: a physiopathologic evaluation. Ann Surg 2014;260:909-14; discussion 914.
43. Coupaye M, Gorbatchef C, Calabrese D, et al. Gastroesophageal reflux after sleeve gastrectomy: a prospective mechanistic study. Obes Surg 2018;28:838-45.
44. Del Genio G, Tolone S, Limongelli P, et al. Sleeve gastrectomy and development of “de novo” gastroesophageal reflux. Obes Surg 2014;24:71-7.
45. Tolone S, Savarino E, de Bortoli N, et al. Esophageal high-resolution manometry can unravel the mechanisms by which different bariatric techniques produce different reflux exposures. J Gastrointest Surg 2020;24:1-7.
46. Yormaz S, Yılmaz H, Ece I, Yılmaz F, Sahin M. Midterm clinical outcomes of antrum resection margin at laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg 2017;27:910-6.
47. Borbély Y, Schaffner E, Zimmermann L, et al. De novo gastroesophageal reflux disease after sleeve gastrectomy: role of preoperative silent reflux. Surg Endosc 2019;33:789-93.
48. Ortiz V, Alvarez-Sotomayor D, Sáez-González E, et al. Decreased esophageal sensitivity to acid in morbidly obese patients: a cause for concern? Gut Liver 2017;11:358-62.
49. Carabotti M, Avallone M, Cereatti F, et al. Usefulness of upper gastrointestinal symptoms as a driver to prescribe gastroscopy in obese patients candidate to bariatric surgery. A prospective study. Obes Surg 2016;26:1075-80.
50. Ortiz V, Ponce M, Fernández A, et al. Value of heartburn for diagnosing gastroesophageal reflux disease in severely obese patients. Obesity (Silver Spring) 2006;14:696-700.
51. Heimgartner B, Herzig M, Borbély Y, Kröll D, Nett P, Tutuian R. Symptoms, endoscopic findings and reflux monitoring results in candidates for bariatric surgery. Dig Liver Dis 2017;49:750-6.
52. Peterson HR, Rothschild M, Weinberg CR, Fell RD, McLeish KR, Pfeifer MA. Body fat and the activity of the autonomic nervous system. N Engl J Med 1988;318:1077-83.
53. Soliman H, Coupaye M, Cohen-Sors B, et al. Do preoperative esophageal ph monitoring and high-resolution manometry predict symptoms of GERD after sleeve gastrectomy? Obes Surg 2021;31:3490-7.
54. Kristo I, Paireder M, Jomrich G, et al. Silent gastroesophageal reflux disease in patients with morbid obesity prior to primary metabolic surgery. Obes Surg 2020;30:4885-91.
55. Greilsamer T, de Montrichard M, Bruley des Varannes S, et al. Hypotonic low esophageal sphincter is not predictive of gastroesophageal reflux disease after sleeve gastrectomy. Obes Surg 2020;30:1468-72.
56. Navarini D, Madalosso CAS, Tognon AP, Fornari F, Barão FR, Gurski RR. Predictive factors of gastroesophageal reflux disease in bariatric surgery: a controlled trial comparing sleeve gastrectomy with gastric bypass. Obes Surg 2020;30:1360-7.
57. Funk LM, Kanji A, Scott Melvin W, Perry KA. Elective antireflux surgery in the US: an analysis of national trends in utilization and inpatient outcomes from 2005 to 2010. Surg Endosc 2014;28:1712-9.
58. Wang YR, Dempsey DT, Richter JE. Trends and perioperative outcomes of inpatient antireflux surgery in the United States, 1993-2006. Dis Esophagus 2011;24:215-23.
59. Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822-32.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Soricelli E, Facchiano E, Casella G, Genco A, Lucchese M. Which is the best algorithm for evaluating a patient's candidate to sleeve with suspected reflux or hiatal hernia: is manometry or reflux assessment always necessary. Mini-invasive Surg 2022;6:54. http://dx.doi.org/10.20517/2574-1225.2022.32
AMA Style
Soricelli E, Facchiano E, Casella G, Genco A, Lucchese M. Which is the best algorithm for evaluating a patient's candidate to sleeve with suspected reflux or hiatal hernia: is manometry or reflux assessment always necessary. Mini-invasive Surgery. 2022; 6: 54. http://dx.doi.org/10.20517/2574-1225.2022.32
Chicago/Turabian Style
Soricelli, Emanuele, Enrico Facchiano, Giovanni Casella, Alfredo Genco, Marcello Lucchese. 2022. "Which is the best algorithm for evaluating a patient's candidate to sleeve with suspected reflux or hiatal hernia: is manometry or reflux assessment always necessary" Mini-invasive Surgery. 6: 54. http://dx.doi.org/10.20517/2574-1225.2022.32
ACS Style
Soricelli, E.; Facchiano E.; Casella G.; Genco A.; Lucchese M. Which is the best algorithm for evaluating a patient's candidate to sleeve with suspected reflux or hiatal hernia: is manometry or reflux assessment always necessary. Mini-invasive. Surg. 2022, 6, 54. http://dx.doi.org/10.20517/2574-1225.2022.32
About This Article
Copyright
Data & Comments
Data

 Cite This Article 4 clicks
Cite This Article 4 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.