Robotic resection of hilar cholangiocarcinoma: a single institution experience
Abstract
Aim: Hilar cholangiocarcinoma is an aggressive malignancy with a poor prognosis, for which only surgical resection offers potential cure. Because of its complex location in the porta hepatis, the standard surgical approach has been open surgery. With the gradual increase in the use of minimally invasive surgery, we aimed to describe our single institutional experience of robotic resection of hilar cholangiocarcinoma. To the best of our knowledge, this is the largest published series in North America.
Methods: Between 2016-2022, we prospectively followed all patients who underwent robotic extrahepatic biliary resection for hilar cholangiocarcinoma.
Results: Robotic resection of hilar cholangiocarcinoma was performed on 21 patients of median age 72 years, 16 (76%) of whom underwent concomitant hepatectomy. All patients initially presented with jaundice and underwent preoperative drainage. Median operative time was 458 minutes and the estimated blood loss was 150 mL. There were no intraoperative complications or conversions to open surgery. The length of stay was five days, with one readmission at 30 days. There were three postoperative complications and one postoperative mortality (at 90 days). R0 was attained in 90% (19/21) of cases and R1 in 10% (2/21). Our median follow-up time was 21 months. At the final follow-up, 15 patients were alive with no evidence of disease and six died.
Conclusion: Robotic resection of hilar cholangiocarcinoma is safe and feasible and achieves excellent outcomes. We believe that robotic surgery will soon be an accepted approach for complex hepatobiliary resections, such as for hilar cholangiocarcinoma.
Keywords
INTRODUCTION
Hilar cholangiocarcinoma (Klatskin tumor) is a rare and highly aggressive malignancy with a poor prognosis[1,2]. Surgical resection is the only potentially curative treatment modality and achieves the best long-term survival; however, hilar cholangiocarcinoma is one of the most challenging cancers to treat[3]. Many factors prejudice the treatment of this complex disease, including its critical location at the confluence of the bile duct, its aggressive tendency to involve adjacent structures, including major blood vessels, and its pattern of growth along the biliary tree, which makes it difficult to determine the extent of the disease. Additionally, resection of hilar cholangiocarcinoma requires systematic portal lymphadenectomy and complex bilioenteric reconstruction[4,5]. Given these features, a standard open approach is advocated by most hepatobiliary surgeons. Some even consider that a minimally invasive approach to hilar cholangiocarcinoma resection is contraindicated.
The advent of minimally invasive liver surgery (MLS) has marked a new era in hepatobiliary surgery. Over the past two decades, MLS has been increasingly performed and has achieved better outcomes than an open approach[6-8]. A laparoscopic approach to hilar cholangiocarcinoma has been marginally adopted and reported in small numbers, mostly from China[9,10]. Robotic liver surgery has gone one step further in that the benefits inherent in using this system have made it possible to perform both the most complex liver resections and high biliary reconstruction. Previous studies on robotic hepatectomy with biliary reconstruction for hilar cholangiocarcinoma are limited to small series and case reports; these have shown that this approach is safe and feasible[11-13]. The largest Western series reported to date included only four patients[14]. We therefore report here our single institutional experience of 21 patients who underwent robotic resection of hilar cholangiocarcinoma. To the best of our knowledge, this is the largest robotic series ever reported in the Western hemisphere. Our hypothesis was that robotic resection of hilar cholangiocarcinoma can be performed safely with excellent postoperative outcomes.
METHODS
With institutional review board (IRB) approval, from September 2016 through April 2022, we prospectively followed 21 consecutive patients who had undergone robotic extrahepatic biliary resection and reconstruction for hilar cholangiocarcinoma, with or without hepatectomy. Preoperative diagnoses of hilar cholangiocarcinoma were based on clinical findings, high-quality imaging, advanced endoscopy with cholangiography, and cholangioscopic biopsy in the majority of cases. Patients with intrahepatic cholangiocarcinoma, benign or premalignant biliary diseases, and distal cholangiocarcinoma were excluded from the study.
Patient characteristics and other clinical data collected and analyzed included age, sex, Body Mass Index (BMI), American Society of Anesthesiology (ASA) score, Childs-Pugh score, Model for End-Stage Liver Disease (MELD) score, tumor size, jaundice on presentation, preoperative biliary drainage, preoperative positive biopsy, neoadjuvant treatment, jaundice on day of surgery, operative duration, estimated blood loss (EBL), Bismuth-Corlette Classification, concomitant hepatectomy, intraoperative complications, lymph nodes removed, lymph node positivity, margin status, pathological type, postoperative complications according to the Clavien-Dindo classification, length of stay (LOS), 30-day readmission, in-hospital mortality, 90-day mortality, follow-up time, and current oncological status.
The preoperative workup included a triple phase 1-mm cut computed tomography (CT) scan of the abdomen and pelvis, chest CT, and abdominal magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP). This high-quality imaging is mainly performed to evaluate the blood vessels (portal vein and hepatic artery) along the porta hepatis and to assess any aberrant vasculature and/or vessel involvement. MRCP is performed to map the biliary tree, assess the extent of intrahepatic sectoral biliary obstruction, and plan reconstruction of the biliary tree after completion of resection. Future liver remnant volume was calculated on the basis of imaging findings in patients who required extended major hepatic resection. Patients with cholangitis, hyperbilirubinemia > 3 mg/dL, or who required a major hepatectomy with borderline future liver remnant volume underwent preoperative biliary drainage. In most cases, drainage was achieved by endoscopic retrograde cholangiopancreatography (ERCP) with brushing and stent placement. Many of the patients had already undergone this procedure prior to referral to our center. When available and necessary, cholangioscopy with biopsy was added. Percutaneous transhepatic cholangiography (PTC) was performed when bilirubin concentrations remained high following ERCP stenting or when ERCP was not technically feasible. In addition, every patient was clinically evaluated in terms of performance status, medical comorbidities, and cardiac and liver function.
Operative procedure
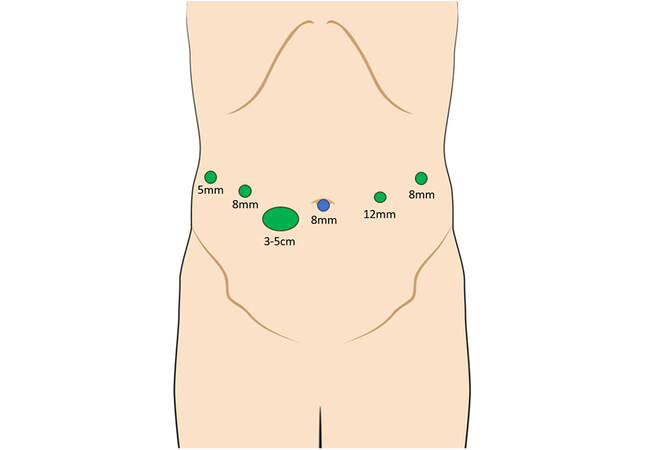
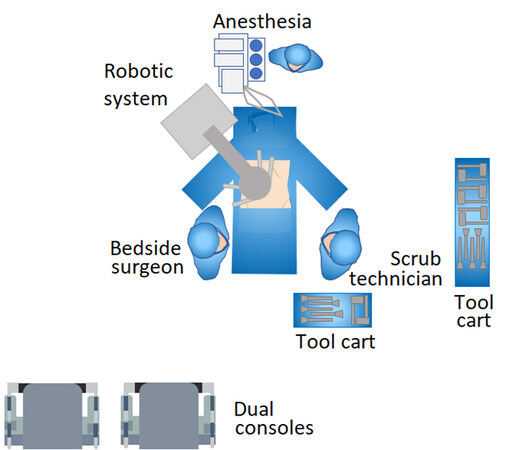
All operations were performed using the da Vinci Xi® robotic surgical system (Intuitive Surgical, Sunnyvale, CA, USA). Exclusion criteria for the robotic approach comprised hepatic artery and/or main portal vein invasion that required major vascular resection and reconstruction. Patients were placed in a supine position with both hands abducted to less than 90° on arm boards. Following the insertion of an 8-mm port through the umbilicus, the procedure commenced with diagnostic laparoscopy. After excluding peritoneal spread, an 8-mm port was placed along the right midclavicular line and a 12-mm port along the left midclavicular line, both at the level of the umbilicus. A fourth 8-mm port was placed in the left anterior axillary line. Finally, an Advanced Access Gelport® (Applied Medical, Rancho Santa Margarita, CA, USA) was placed between the right midclavicular line and umbilicus, slightly inferior to the umbilicus. The Gelport® is used by the bedside surgeon, mainly for suctioning and insertion of sutures. The incision
Data were documented on an Excel (Microsoft) spreadsheet and analyses were carried out using GraphPad Prism 8TM software (GraphPad Software, San Diego, CA, USA). The data are presented as median
RESULTS
During the study period, 21 patients underwent robotic extrahepatic biliary resection, with or without hepatectomy, for hilar cholangiocarcinoma. The median age was 72 (71 ± 8.3) years; 14 (67%) were men and seven (33%) were women. The median BMI was 25 (27 ± 5.9) kg/m2, Child-Pugh score 5 (6 ± 1.0), and MELD score 9 (11 ± 6.0). All patients initially presented with jaundice and underwent preoperative biliary drainage; 15 (71%) patients had a stent placed by ERCP, one (5%) was stented by PTC, and five (24%) required both ERCP and PTC. Positive biopsies were obtained in 17 (81%) patients, three (24%) of whom were referred for neoadjuvant treatment before resection. On the day of operation, 13 (62%) patients had persistent, mostly mild, hyperbilirubinemia (1-3 mg/dL). Four patients had persistent hyperbilirubinemia with bilirubin > 3 mg/dL despite all drainage efforts [Table 1].
Preoperative patient characteristics and clinical data
| Variables | Number |
| Number of patients | 21 |
| Age (years) | 72 (72 ± 8.6) [55-90] |
| Sex (M/W) | 14M/7W |
| BMI (kg/m2) | 25 (27 ± 5.9) [19-40] |
| ASA class | 3 (3 ± 0.4) |
| Childs-pugh score | 5 (6 ± 1.0) |
| MELD score | 9 (11 ± 6.0) |
| Tumor size (cm) | 2 (2 ± 1.2) [0.7-3] |
| Jaundice at presentation | 21 (100%) |
| Preoperative biliary drainage | 21 (100%) |
| ERCP drainage (n) | 15 (71%) |
| PTC drainage (n) | 1 (5%) |
| ERCP and PTC drainage (n) | 5 (24%) |
| Preoperative positive biopsy (n) | 17 (81%) |
| Neoadjuvant therapy (n) | 3 (14%) |
| Jaundiced on day of surgery | 13 (62%) |
| Bilirubin 1-3 mg/dL | 9 (69%) |
| Bilirubin > 3 mg/dL | 4 (31%) |
According to the Bismuth classification, four patients had Type I disease, five Type II, 10 Type III, and two Type IV. Sixteen patients (76%) required concomitant hepatectomy; one underwent right hepatectomy, seven left hepatectomy, and eight central hepatectomy. Operative time was 458 (433 ± 116.9) minutes with an EBL of 150 (175 ± 123.8) mL. There were no intraoperative complications and no conversions to open surgery. Final pathological outcomes were as follows: four (5 ± 2.9) lymph nodes were examined per case, 0 (0 ± 0.4) lymph nodes were positive, R0 was attained in 90% (19/21) of cases and R1 in 10% (2/21) [Table 2].
Intraoperative variables
| Variables | Number |
| Operative duration (minutes) | 458 (433 ± 116.9) [279-573] |
| EBL (mL) | 150 (175 ± 123.8) [10-450] |
| Bismuth-corlette classification | |
| Type I | 4 |
| Type II | 5 |
| Type III | 10 |
| Type IV | 2 |
| Concomitant hepatectomy | 16 (76%) |
| Right hepatectomy | 1 (6%) |
| Left hepatectomy | 7 (44%) |
| Central hepatectomy | 8 (50%) |
| Intraoperative complications (n) | 0 |
| Lymph nodes harvested (n) | 4 (5 ± 2.9) [1-12] |
| Lymph nodes positive (n) | 0 (0 ± 0.4) [0-2] |
| Margin status | |
| R0 | 19 (90%) |
| R1 | 2 (10%) |
| R2 | 0 |
Following stratification according to the Bismuth classification, there were no statistically significant differences between operative times for different Bismuth types (P = 0.69). The EBL for Bismuth Types I/II/III/IV was 275/100/150/113 mL, respectively (P = 0.79). The R1 resection rate was 25% (1/4) for Bismuth Type I and 20% (1/5) for Bismuth Type II. In the two cases in which R1 resection was not achieved, the margins were reported as negative on frozen section; however, the final pathological examination revealed microscopic involvement of the margins. All procedures on patients with Bismuth Types III and IV achieved clear margins [Table 3].
Intraoperative variables stratified by Bismuth classification
| Bismuth classification | Type I n = 4 | Type II n = 5 | Type III n = 10 | Type IV n = 2 | P-value |
| Operative time (minutes) | 453 (416 ± 93.0) | 383 (383 ± 55.0) | 518 (453 ± 158.0) | 486 (486 ± 40.0) | 0.69 |
| EBL (mL) | 275 (225 ± 119) | 100 (180 ± 160) | 150 (166 ± 125) | 113 (113 ± 124) | 0.79 |
| Margin status (r0/r1/r2) | 3/1/0 | 4/1/0 | 10/0/0 | 2/0/0 | 0.36 |
| Lymph node harvested (n) | 6 (7 ± 4.1) | 3 (3 ± 2.6) | 5 (5 ± 2.6) | 3 (3 ± 1.4) | 0.33 |
| Lymph nodes positive (n) | 1 (1 ± 1.0) | 0 (0 ± 0.4) | 0 (0 ± 0.3) | 0 (0 ± 0) | 0.20 |
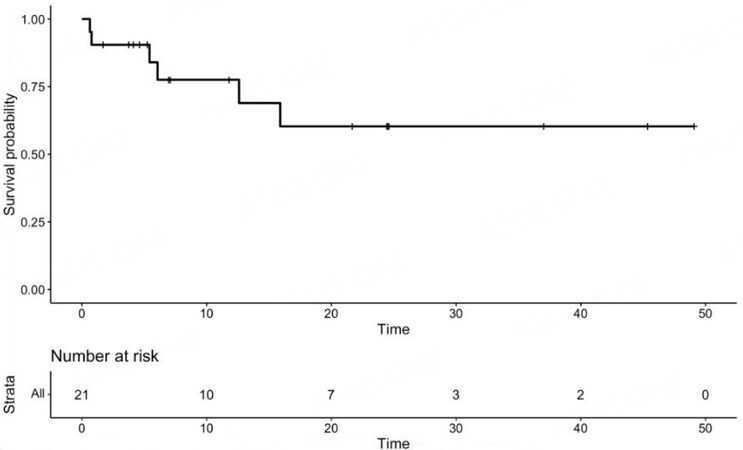
Overall, there were three postoperative complications. Two patients developed intra-abdominal fluid collections that required intravenous antibiotics and percutaneous drainage. One of them, a 90-year-old patient with emphysema, was discharged on postoperative Day 12 and died within 90 days of respiratory failure. Another patient developed a small pneumothorax, probably due to a central line placed preoperatively. The pneumothorax resolved spontaneously without the need for tube thoracostomy. Median LOS was 5 (6 ± 3.4) days with one readmission at 30 days. There were no in-hospital mortalities. At a median follow-up time of 21 months, 15 patients were alive with no evidence of disease and six had died [Table 4]. The median overall survival had not been reached at the time of analysis. The one-year survival rate was 78% and the three- and five-year survival rates were both 60% [Figure 3].
Postoperative variables
| Variables | Number |
| Postoperative complications (n) | 3 |
| Pneumothorax | 1 |
| Intra-abdominal fluid collection | 2 |
| Clavien-dindo classification | |
| Grade II | 1 |
| Grade III | 2 |
| Length of stay (days) | 5 (6 ± 3.4) [3-14] |
| 30-day readmission (n) | 1 |
| In-hospital mortality (n) | 0 |
| 90-day mortality | 1 |
| Follow-up time (months) | 21 (22 ± 14.9) [1-63] |
| Current status | |
| Alive without disease (n) | 15 |
| Alive with disease (n) | 0 |
| Deceased (n) | 6 |
DISCUSSION
Hilar cholangiocarcinoma is one of the most complex malignancies to diagnose and treat. Its characteristic clinical presentation of obstructive jaundice with intrahepatic bile duct dilation usually requires preoperative drainage to resolve the cholangitis and improve hepatic function. However, unlike pancreatic head cancer or distal cholangiocarcinoma, where the drainage procedure is usually straightforward, in hilar cholangiocarcinoma, drainage can be challenging, requiring multiple biliary drains to achieve decompression and prevent the development of a cholestatic liver. In addition, it is often difficult to obtain a positive biopsy and determine the full extent of biliary involvement, this sometimes only being determined intra-operatively. The aggressive biology of this tumor is a major contributor to its complexity in that adjacent structures, such as lymph nodes and major vessels, are frequently involved, often necessitating meticulous lymph node dissection and a major liver resection, with or without vascular reconstruction. Finally, its critical location at the confluence of the bile duct, together with its propensity to grow along the biliary tree, may result in a difficult and high bilioenteric reconstruction. These complex considerations have resulted in most surgeons using a traditional open approach. Our hepatopancreatobiliary unit has gained considerable experience in robotic liver and pancreas surgery over the past six years. Having extensive experience in robotic pancreatectomy enabled us to successfully implement a robotic surgical platform for biliary and hepatic surgery. We started with simple procedures such as peripheral anterolateral segmentectomy and left lateral hepatectomy, then gradually progressed to more complex procedures such as formal left hepatectomy, caudate lobe resection, and formal right hepatectomy, ultimately achieving the most challenging procedures, including robotic resection of hilar cholangiocarcinoma. To the best of our knowledge, this series of 21 patients who underwent robotic extrahepatic bile duct cancer resection with or without concomitant hepatectomy is the largest robotic series reported in the Western world so far. Our results support our hypothesis that robotic resection for hilar cholangiocarcinoma is safe and achieves excellent postoperative outcomes.
Most of our patients were men in their 70s. All patients presented with obstructive jaundice and underwent a preoperative drainage procedure, in most cases by ERCP. Preoperative biliary drainage and the best modality to use (endoscopic via ERCP vs. percutaneous via PTC) are well-known controversial issues in the management of hilar cholangiocarcinoma; however, preoperative biliary drainage is required prior to most resections[17]. We have chosen to utilize ERCP for the following reasons. First, our center has a highly skilled and experienced advanced endoscopy unit that is capable of successfully performing this procedure with minimal complications. Second, performing cholangiography is extremely important in clarifying the anatomy of the biliary tree and it provides better quality information than MRCP. Third, obtaining a positive biopsy is important, especially before embarking on a complex surgery with the potential for high morbidity. In recent years, the use of ERCP with cholangioscopy has resulted in significant improvement in diagnostic accuracy with minimal morbidity. We achieved positive preoperative biopsies in 81% of our cases, similar to a previous multicenter study[18]. Fourth, preoperative drainage of the future liver remnant is necessary for patients who require major hepatectomy because significant cholestasis can impair liver function and regeneration following major liver resection[19]. Despite all our drainage efforts, on the day of surgery, four patients still had bilirubin concentrations of > 3 mg/dL.
The operative time was 458 (433 ± 116.9) minutes and the EBL was 150 (175 ± 123.8) mL. Our results are superior to other studies on robotic resection of hilar cholangiocarcinoma. A previous series of 10 patients by Xu et al. reported an operative time of 703 ± 62 minutes with intraoperative blood loss of
As to oncological outcomes, we achieved an R0 resection rate of 90% and a survival rate of 71% over a
In summary, we here present our initial series of robotic resections of hilar cholangiocarcinoma. Our results indicate that with adequate surgical experience, this operation can be performed safely with excellent outcomes. The main limitation of this study is that it was not a comparative study: we did not compare our results with those of an open surgery control group. Another limitation was the small cohort of 21 patients, which is unsurprising given the rarity of this disease. However, this is the largest published Western cohort of patients undergoing robotic surgery for Klatskin tumors and it presents various benefits in perioperative outcomes without compromising oncological outcomes. As this technology keeps growing and advancing, we believe that the use of a robotic platform will become an alternative approach, not only for major liver operations but also for resection of hilar cholangiocarcinoma.
DECLARATIONS
Authors’ contributionsMade substantial contributions to the conception and design of the study and performed data analysis and interpretation: Jacoby H, Rayman S, Ross S, Rosemurgy A, Sucandy I
Performed data acquisition and data analysis, and provided administrative, technical, and material support: Crespo K, Syblis C
Availability of data and materialsNot applicable.
Financial support and sponsorshipNot applicable.
Conflicts of interestRoss S: Reports educational (personal fees and non-financial support) relationship with Intuitive Surgical Incorporated, outside the submitted work. All other authors declared that there are no conflicts of interest.
Ethical approval and consent to participateAll participants were properly consented, and the study was approved by the IRB (1302427).
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Launois B, Reding R, Lebeau G, Buard JL. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg 2000;7:128-34.
2. Nakeeb A, Pitt HA, Sohn TA. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463.
3. Dondossola D, Ghidini M, Grossi F, Rossi G, Foschi D. Practical review for diagnosis and clinical management of perihilar cholangiocarcinoma. World J Gastroenterol 2020;26:3542-61.
4. Anderson B, Doyle MBM. Surgical considerations of hilar cholangiocarcinoma. Surg Oncol Clin N Am 2019;28:601-17.
5. Cipriani F, Ratti F, Fiorentini G, Reineke R, Aldrighetti L. Systematic review of perioperative and oncologic outcomes of minimally-invasive surgery for hilar cholangiocarcinoma. Updates Surg 2021;73:359-77.
6. Duarte VC, Coelho FF, Valverde A, et al. Minimally invasive versus open right hepatectomy: comparative study with propensity score matching analysis. BMC Surg 2020;20:260.
7. Goh BKP, Syn N, Koh YX, et al. Comparison between short and long-term outcomes after minimally invasive versus open primary liver resections for hepatocellular carcinoma: a 1:1 matched analysis. J Surg Oncol 2021;124:560-71.
8. Schiffman SC, Kim KH, Tsung A, Marsh JW, Geller DA. Laparoscopic versus open liver resection for metastatic colorectal cancer: a metaanalysis of 610 patients. Surgery 2015;157:211-22.
9. Liu S, Liu X, Li X, et al. Application of laparoscopic radical resection for type III and IV hilar cholangiocarcinoma treatment. Gastroenterol Res Pract 2020;2020:1506275.
10. Wang W, Fei Y, Liu J, Yu T, Tang J, Wei F. Laparoscopic surgery and robotic surgery for hilar cholangiocarcinoma: an updated systematic review. ANZ J Surg 2021;91:42-8.
11. Machado MA, Mattos BV, Lobo Filho MM, Makdissi F. Robotic resection of hilar cholangiocarcinoma. Ann Surg Oncol 2020;27:4166-70.
12. Camerlo A, Seux H, Fara R. Robotic left hepatectomy extended to caudate lobe and common biliary duct for hilar cholangiocarcinoma. Ann Surg Oncol 2022;29:2407.
13. Sucandy I, Ross S, Rosemurgy A. Robotic resection of a type IIIB klatskin tumor. J Gastrointest Surg 2021;25:1939-40.
14. Cillo U, D'Amico FE, Furlanetto A, Perin L, Gringeri E. Robotic hepatectomy and biliary reconstruction for perihilar cholangiocarcinoma: a pioneer western case series. Updates Surg 2021;73:999-1006.
15. Sucandy I, Gravetz A, Ross S, Rosemurgy A. Technique of robotic left hepatectomy : how we approach it. J Robot Surg 2019;13:201-7.
16. Sucandy I, Durrani H, Ross S, Rosemurgy A. Technical approach of robotic total right hepatic lobectomy: how we do it? J Robot Surg 2019;13:193-9.
17. Soares KC, Jarnagin WR. The landmark series: hilar cholangiocarcinoma. Ann Surg Oncol 2021;28:4158-70.
18. Navaneethan U, Hasan MK, Kommaraju K, et al. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: a multicenter clinical experience (with video). Gastrointest Endosc 2016;84:649-55.
19. Lidsky ME, Jarnagin WR. Surgical management of hilar cholangiocarcinoma at Memorial Sloan Kettering Cancer Center. Ann Gastroenterol Surg 2018;2:304-12.
20. Xu Y, Wang H, Ji W, et al. Robotic radical resection for hilar cholangiocarcinoma: perioperative and long-term outcomes of an initial series. Surg Endosc 2016;30:3060-70.
21. Tang W, Qiu JG, Deng X, et al. Minimally invasive versus open radical resection surgery for hilar cholangiocarcinoma: comparable outcomes associated with advantages of minimal invasiveness. PLoS One 2021;16:e0248534.
22. Ma D, Wang W, Wang J, et al. Laparoscopic versus open surgery for hilar cholangiocarcinoma: a retrospective cohort study on short-term and long-term outcomes. Surg Endosc 2022;36:3721-31.
23. Wiggers JK, Groot Koerkamp B, Cieslak KP, et al. Postoperative mortality after liver resection for perihilar cholangiocarcinoma: development of a risk score and importance of biliary drainage of the future liver remnant. J Am Coll Surg 2016;223:321-31.e1.
24. Kang MJ, Jang JY, Chang J, et al. Actual long-term survival outcome of 403 consecutive patients with hilar cholangiocarcinoma. World J Surg 2016;40:2451-9.
25. Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 2013;258:129-40.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Jacoby H, Rayman S, Ross S, Crespo K, Syblis C, Rosemurgy A, Sucandy I. Robotic resection of hilar cholangiocarcinoma: a single institution experience. Mini-invasive Surg 2022;6:58. http://dx.doi.org/10.20517/2574-1225.2022.58
AMA Style
Jacoby H, Rayman S, Ross S, Crespo K, Syblis C, Rosemurgy A, Sucandy I. Robotic resection of hilar cholangiocarcinoma: a single institution experience. Mini-invasive Surgery. 2022; 6: 58. http://dx.doi.org/10.20517/2574-1225.2022.58
Chicago/Turabian Style
Jacoby, Harel, Shlomi Rayman, Sharona Ross, Kaitlyn Crespo, Cameron Syblis, Alexander Rosemurgy, Iswanto Sucandy. 2022. "Robotic resection of hilar cholangiocarcinoma: a single institution experience" Mini-invasive Surgery. 6: 58. http://dx.doi.org/10.20517/2574-1225.2022.58
ACS Style
Jacoby, H.; Rayman S.; Ross S.; Crespo K.; Syblis C.; Rosemurgy A.; Sucandy I. Robotic resection of hilar cholangiocarcinoma: a single institution experience. Mini-invasive. Surg. 2022, 6, 58. http://dx.doi.org/10.20517/2574-1225.2022.58
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 11 clicks
Cite This Article 11 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.