Minimally invasive spleen-preserving distal pancreatectomy: real-world data from the Italian National Registry of Minimally Invasive Pancreatic Surgery (IGoMIPS)
Abstract
Aim: Minimally invasive distal pancreatectomy has become the standard of care for benign and low malignant lesions. Spleen preservation in this setting has been proposed to reduce surgical trauma and long-term sequelae. The aim of the current study is to present real-world data on indications, techniques, and outcomes of spleen-preserving distal pancreatectomy (SPDP).
Methods: Patients who underwent SPDP and distal pancreatectomy with splenectomy (DPWS) were extracted from the 2019-2022 Italian National Registry for Minimally Invasive Pancreatic Surgery (IGoMIPS). Perioperative and pathological data were collected.
Results: One hundred and ten patients underwent SPDP and five hundred and seventy-eight underwent DPWS. Patients undergoing SPDP were significantly younger (56 vs. 63.5 years; P < 0.001). Seventy-six percent of SPDP cases were performed in six out of thirty-four IGoMIPS centers. SPDP was performed predominantly for Neuroendocrine Tumors (43.6% vs.23.5%; P < 0.001) and for smaller lesions (T1 57.6% vs. 29.8%; P < 0.001). The conversion rate was higher in the case of DPWS (7.6% vs. 0.9%; P = 0.006), even when pancreatic cancer was ruled out (5.0% vs. 0.9%; P = 0.045). The robotic approach was most commonly used for SPDP (50.9% vs. 29.7%; P < 0.001). No difference in postoperative outcomes and length of stay was observed between the two groups, as well as between robotic and laparoscopic approaches in the SPDP group. A trend toward a lower rate of postoperative sepsis was observed after SPDP (0.9% vs. 5.2%; P = 0.056). In 84.7% of SPDP, splenic vessels were preserved (Kimura procedure) without an impact on short-term postoperative outcomes.
Conclusion: In this registry analysis, SPDP was feasible and safe. The Kimura procedure was prevalent over the Warshaw procedure. The typical patient undergoing SPDP was young with a neuroendocrine tumor at an early stage. Robotic assistance was used more frequently for SPDP than for DPWS.
Keywords
INTRODUCTION
Minimally invasive distal pancreatectomy has become the standard of care for benign and low malignant lesions of body and tail of the pancreas[1,2]. Portended advantages of spleen preservation include prevention of overwhelming sepsis and thrombocytosis, as well as preserved overall immune function[3-5]. Some authors argued that spleen preservation may reduce blood loss and operative time while improving postoperative outcomes such as postoperative pancreatic fistula occurrence and delayed gastric emptying[3,6-8]. However, the literature appears rather heterogenous, and high-level evidence on the real advantages of spleen preservation is still lacking. On practical grounds, the decision of whether to preserve the spleen is surgeon-dependent.
From a technical point of view, the spleen can be preserved either with the splenic vessels (Kimura technique)[9] or with the en bloc resection of the splenic vessels (Warshaw technique)[10]. In the Warshaw technique, the spleen is supplied from collateral circulation with an increased risk of early splenic infarction and late left portal hypertension.
The Italian National Registry for Minimally Invasive Pancreatic Surgery (IGoMIPS)[11,12] was founded in 2019. To date, 1,300 minimally invasive pancreatic resections have been prospectively reported to the registry by 34 Italian centers.
This study aims to report real-world data on indications, techniques, and intra- and postoperative outcomes of spleen-preserving distal pancreatectomy (SPDP).
METHODS
The registry
The IGoMIPS Registry was established in 2019. Free access to the Registry is granted to all Italian centers performing minimally invasive pancreatic surgery following bylaws approval by the local Ethical Committee. In order to capture data on adherence to the planned approach, patients must be enrolled in the Registry the day before surgery.
To be eligible for inclusion into the registry, each patient must be ≥ 18 years old and must have signed an informed consent. Operative and postoperative variables are collected for all patients during the first 90 postoperative days. IGoMIPS is recorded in the Registry of Patient Registries (RoPR) of the Agency for Healthcare and Research and Quality, US Department of Health (Registry of Patient Registries. Content last reviewed April 2019. https://www.ahrq.gov/ropr/index.html. The registry protocol is approved by the Independent Ethics Committee of the Humanitas Institute (Authorization Number 2,167).
At the access to the registry, the surgeons performing the analyzed procedures presented a median [IQR] caseload for open pancreatic resection, minimally invasive major procedures and specifically pancreatic procedures of 392 [485], 92 [117], and 705 [1,070], respectively.
Study design
This study aims to present data from SPDP prospectively enrolled in IGoMIPS. A comparison with distal pancreatectomy with en-bloc splenectomy (DPWS) and a subgroup analysis comparing the outcomes of Kimura[9] and Warshaw[10] techniques are also presented.
Data collection
Collected data include identifiers and characteristics of the enrolling center (including progressive case number), caseload of the first and second operating surgeon, patient characteristics, details of surgical procedure, type of minimally invasive approach (i.e., laparoscopic, robotic, hand-assisted, etc.), intraoperative data, postoperative outcomes, histo-pathological data, and long-term follow-up.
Data extraction
Data from all patients scheduled for SPDP and DPWS were extracted from the registry.
Data analysis
A per-protocol analysis was performed for patients who actually underwent SPDP, including those who were planned for a different procedure (e.g., middle pancreatectomy or DPWS).
Statistics
Statistical computations were performed using the software STATA 17.0 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.). Descriptive and inferential statistics were carried out with the analytical models adequate for the type of variable studied (e.g., Mann-Whitney test, chi- square). Two-sided P values lower than 0.05 were considered statistically significant. All variables were reported as the median and interquartile range (IQR).
RESULTS
As of June 2022, 1,293 minimally invasive pancreatic resections were enrolled in IGoMIPS. Twenty-one centers reported at least one SPDP (range 1-27; median [IQR]: 2 [7]). DPWS was reported by all thirty-four centers (range 1-123; median [IQR]: 9 [16]). Seventy-six percent of all SPDP procedures were performed in six of the participating centers.
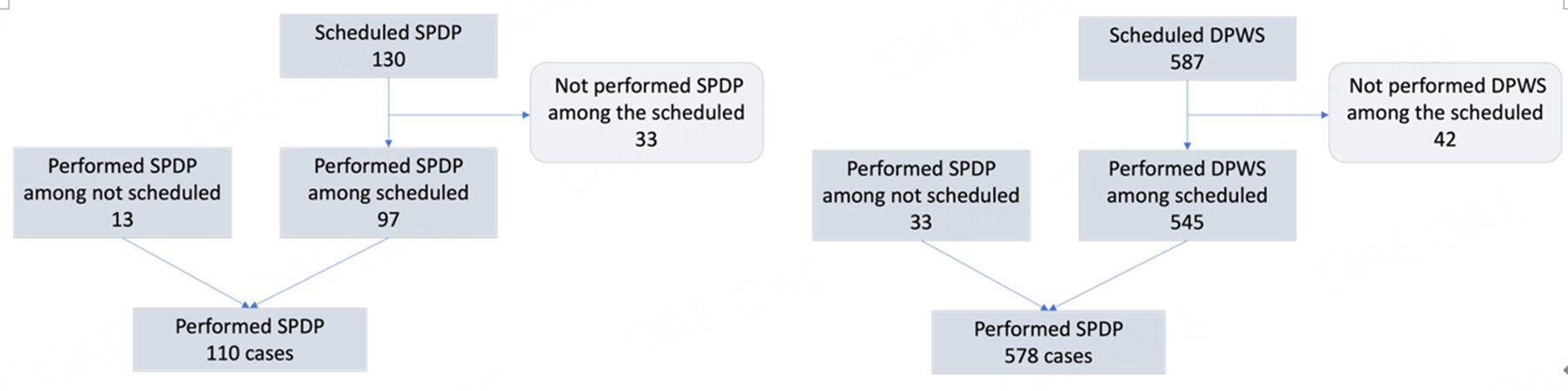
SPDP was performed in 97 of 130 patients planned for this procedure (74.6%). The remaining patients underwent DPWS (n = 25; 19.2%), tumor enucleation (n = 4; 3.0%), middle pancreatectomy (n = 2; 1.5%), or exploratory laparoscopy (n = 2; 1.5%).
Thirteen additional SPDP were performed in patients scheduled for DPWS (n = 6), tumor enucleation
Overall, there were 110 SPDP, with one conversion to open surgery (0.9%). Data on preservation of splenic vessels was available in 92 cases: a Kimura procedure was performed in 78 patients (84.7%) and a Warshaw procedure in 14 patients (15.2%).
During the same period, 587 procedures were pre-registered as DPWS and 545 actually underwent that procedure (92.8%). The remaining patients underwent exploratory laparoscopy (n = 16; 2.7%), tumor enucleation (n = 9; 1.5%), SPDP (n = 6; 1.0%), total pancreatectomy (n = 6; 1.0%), and open pancreatoduodenectomy (n = 1; 0.1%). Finally, 4 patients (0.6%) had a final diagnosis different from a pancreatic tumor and underwent different procedures as required by the final diagnosis.
Overall, 578 DPWS were performed, including 545 (94.3%) procedures pre-recorded for that operation and 33 scheduled for a different procedure. Conversion to open surgery was required in 44 patients (7.6%).
The flow-chart of included cases is reported in Figure 1.
Robotic assistance was used in 55 patients undergoing SPDP (50.9%) (laparoscopy: 53 patients; 49.1%) and in 165 patients undergoing DPWS (29.7%) (laparoscopy: 391 patients; 70.3% - P < 0.001). In few patients, the approach was either hybrid or unspecified.
Pathology data were available for 103 SPDP (93.6%). Interestingly, the final diagnosis showed a primary malignant tumor of the pancreas in 5 patients (4.8%). Three patients had a mucinous cystadenocarcinoma and 2 had a pancreatic ductal adenocarcinoma. The final histology is presented in Table 1.
Final histology following spleen-preserving distal pancreatectomy (SPDP)
| Definitive pathological diagnosis | N = 103 |
| Pancreatic neuroendocrine tumor | 48 (46.6%) |
| Intraductal papillary mucinous neoplasm | 14 (13.6%) |
| Solid pseudopapillary tumor | 11 (10.7%) |
| Mucinous cystadenoma | 9 (8.8%) |
| Serous cystadenoma | 7 (6.7%) |
| Mucinous cystadenocarcinoma | 3 (2.9%) |
| Pancreatic ductal adenocarcinoma | 2 (1.9%) |
| Chronic pancreatitis | 2 (1.9%) |
| Pancreatic cysts | 2 (1.9%) |
| Metastasis from renal cell carcinoma | 1 (1.0%) |
| Insulinoma | 1 (1.0%) |
| Accessory spleen | 1 (1.0%) |
| Lymphoepithelial cyst of the pancreas | 1 (1.0%) |
| Traumatic fracture of pancreas | 1 (1.0%) |
Spleen-preserving distal pancreatectomy versus distal pancreatectomy with splenectomy
Population characteristics, intraoperative data, and postoperative outcomes are summarized in Table 2. At baseline, the two groups differed only in median patient age, which was lower for SPDP (56 [42-70] years vs. 63.5 [54-73] years; P = 0.0001). The use of robotic assistance was prevalent in SPDP (50.9% vs. 29.7%; P < 0.0001). Operative time was similar, and a minimal difference was observed in the median estimated blood loss in favor of SPDP (100 [50-189] mL vs. 100 [80-250] mL; P = 0.006). DPWS was associated with higher rates of conversion to open surgery (8% vs. 0.9%; P = 0.008). The difference persisted but became less evident when DPWS performed for pancreatic cancer (n = 233/537; 43.4%) were excluded (5% vs. 0.9%; P = 0.045). However, the need to amend the procedure initially planned was higher for SPDP (n = 33; 25% vs.
Baseline characteristics, intraoperative outcome measures, and early postoperative results of spleen-preserving distal pancreatectomy (SPDP) and distal pancreatectomy with splenectomy (DPWS)
| SPDP (n = 110) | DPWS (n = 578) | P | |
| Median age [IQR], years | 56 [42-70] | 63 [54–73] | 0.0001 |
| BMI, Kg/m2 | 25 [22-29] | 25 [22–28] | 0.649 |
| Female, n | 60 (54.5%) | 311 (53.9%) | 0.901 |
| ASA ≥ 3, n (%) | 34 (31.2%) | 187 (32.6%) | 0.768 |
| Previous abdominal surgery, n (%) | 49 (44.5%) | 260 (45.2%) | 0.897 |
| Minimally invasive technique§ Robotic, n (%) Laparoscopic, n (%) | 55 (50.9%) 53 (49.1%) | 165 (29.7%) 391 (70.3%) | < 0.0001 |
| Median operative time [IQR], minutes | 240 [195-317] | 252 [210-325] | 0.204 |
| Median estimated blood loss [IQR], mL | 100 [50-189] | 100 [80-250] | 0.006 |
| Preservation of splenic vessels° | 78 (84.7%) | - | |
| Pancreatic stump closure n (%)* Stapled Reinforced stapling Suture Harmonic scalpel | 73 (76.8%) 38 (53.1%) 9 (9.5%) 13 (13.7%) | 354 (80.6%) 155 (45%) 43 (9.8%) 42 (9.6%) | 0.488 |
| Associated gastrointestinal tract resections, n (%)# | 0 | 18 (3.2%) | 0.060 |
| Open conversion, n (%) | 1 (0.9%) | 44 (7.6%) | 0.008 |
| Clinically-relevant postoperative pancreatic fistula, n (%)[13] Grade B Grade C | 16 (15.5%) 16 0 | 106 (18.4%) 105 1 | 0.490 |
| Delayed gastric emptying, n (%) | 1 (0.9%) | 10 (1.7%) | 0.592 |
| Post-pancreatectomy hemorrhage, n (%) | 5 (4.8%) | 15 (2.6%) | 0.224 |
| Wound infection, n (%) | 0 | 8 (1.5%) | 0.211 |
| Abdominal collection, n (%) | 17 (16.5%) | 104 (18.0%) | 0.708 |
| Sepsis, n (%) | 1 (0.9%) | 30 (5.2%) | 0.056 |
| Severe postoperative complications (Clavien-Dindo ≥ 3), n (%)[14] | 9 (8.2%) | 60 (10.4%) | 0.482 |
| Reintervention, n (%) | 4 (3.6%) | 12 (2.1%) | 0.261 |
| Readmission, n (%) | 9 (8.2%) | 64 (11.1%) | 0.529 |
| Median length of hospital stay [IQR], days | 7 [5-11] | 7 [6-10] | 0.684 |
| Postoperative mortality, n (%) | 0 | 2 (0.3%) | 0.528 |
| Lesion diameter, cm | 3.0 (2.0-4.2) | 2.3 (1.5-3.5) | 0.0001 |
| Malignant pancreatic lesion | - | 233 (43.4%) | |
| Tumor size, cm# | - | 2.7 (2.0-3.8) | |
| T3-4, n (%)# | - | 48 (25.1%) | |
| RAMPS, n (%)# | - | 101 (43.3%) |
Regarding early postoperative results, the rate of infection at 90 days appeared to be higher following DPWS despite no statistical significance (5.2% vs. 0.9%; P = 0.056).
SPDP was performed more frequently in patients with neuroendocrine tumors (43.6% vs. 23.5%; P < 0.001) at an early stage (T1 57.6% vs. 29.8%; P < 0.001).
Robotic versus laparoscopic spleen-preserving distal pancreatectomy
Population characteristics, intraoperative data, and postoperative outcomes are summarized in Table 3. Baseline characteristics were similar between the two groups. Robotic assistance and conventional laparoscopy were employed equally for SPDP (50.9% vs. 49.1%). The robotic platform was not available in six of the twenty-one centers enrolling SPDP. Excluding these centers, robotic assistance was used in 55.6% of SPDP.
Baseline characteristics, intraoperative outcome measures, and early postoperative results of robotic and laparoscopic spleen-preserving distal pancreatectomy (SPDP)
| Laparoscopic SPDP n = 53 | Robotic SPDP n = 55 | P | |
| Median age [IQR], years | 56 [38-70] | 54 [42-69] | 0.953 |
| BMI, Kg/m2 | 25 [22-28] | 25 [22-29] | 0.433 |
| Female, n | 32 (60.4%) | 28 (50.9%) | 0.322 |
| ASA ≥ 3, n (%) | 19 (35.8%) | 14 (25.9%) | 0.266 |
| Previous abdominal surgery, n (%) | 24 (45.3%) | 25 (45.4%) | 0.986 |
| Median operative time [IQR], minutes | 226.5 [170-315] | 255.0 [220-331] | 0.142 |
| Median estimated blood loss [IQR], mL | 100 [50-200] | 100 [50-150] | 0.227 |
| Preservation of splenic vessels° | 38 (86.4%) | 39 (83.0%) | 0.655 |
| Pancreatic stump closure n (%)* Stapled Reinforced stapling Suture Harmonic scalpel | 39 (86.7%) 23 (63.9%) 2 (4.4%) 4 (8.9%) | 34 (68%) 11(39.3%) 7 (14%) 9 (18%) | 0.091 |
| Conversion to open surgery, n (%) | 1 (1.9%) | 0 | 0.315 |
| Clinically relevant postoperative pancreatic fistula (B/C grade), n (%) | 10 (19.6%) | 6 (11.8%) | 0.276 |
| Delayed gastric emptying, n (%) | 1 (1.9%) | 0 | 0.315 |
| Post-pancreatectomy hemorrhage, n (%) | 3 (5.9%) | 2 (3.9%) | 0.647 |
| Wound infection, n (%) | 0 | 0 | |
| Abdominal collection, n (%) | 12 (23.5%) | 5 (9.8%) | 0.063 |
| Sepsis, n (%) | 1 (1.9%) | 0 | 0.315 |
| Severe postoperative complications (Clavien-Dindo ≥ 3), n (%) [14] | 6 (11.3%) | 3 (5.4%) | 0.270 |
| Reintervention, n (%) | 2 (3.8%) | 2 (3.6%) | 1 |
| Readmission, n (%) | 5 (9.4%) | 4 (7.3%) | 0.727 |
| Median length of hospital stay [IQR], days | 7 [6-12] | 6.5 [5-9] | 0.061 |
| Postoperative mortality, n (%) | 0 | 0 |
Considering all SPDP, there was no difference in histology (P = 0.202) and tumor stage (P = 0.565). No difference was noted in operative time, estimated blood loss, and conversion to open surgery. Regarding early postoperative outcomes, the two groups showed similar incidences of clinically-relevant postoperative pancreatic fistula, post-pancreatectomy hemorrhage, delayed gastric emptying, severe postoperative complications, need for repeat surgery, hospital readmission, and 90-day mortality. Despite the fact that these differences did not reach statistical significance, robotic SPDP indicated a trend toward fewer intra-abdominal fluid collections (9.8% vs. 23.5%; P = 0.063) and a shorter length of hospital stay (6.5 [5-9] days vs. 7 [6-12] days; P = 0.061).
Quite interestingly, the incidence of unplanned splenectomy was similar between the two groups (robotic: 18.5% vs. laparoscopic: 22.7%; P = 0.572).
Preservation of splenic vessels
Kimura SPDP was performed in 78 out of 92 patients (84.7%). Baseline characteristics, intraoperative outcomes, and early postoperative results were comparable between Kimura and Warshaw SPDP [Table 4].
Baseline characteristics, intraoperative outcome measures, and early postoperative results of Kimura and Warshaw SPDP
| ° | Kimura[9] n = 78 | Warshaw[10] n =14 | P |
| Median age [IQR], years | 58 [48-70] | 53 [30-67] | 0.415 |
| BMI, Kg/m2 | 25 [22-28] | 17 [29-29] | 0.403 |
| Female, n | 38 (49.3%) | 14 (71.4%) | 0.128 |
| ASA ≥ 3, n (%) | 27 (35.5%) | 2 (14.3%) | 0.118 |
| Previous abdominal surgery, n (%) | 37 (47.4%) | 5 (35.7%) | 0.394 |
| Median operative time [IQR], minutes | 240 [195-320] | 264.5 [200-423] | 0.483 |
| Median estimated blood loss [IQR], mL | 100 [50-191] | 100 [100-150>] | 0.859 |
| Pancreatic stump closure n (%) Stapled Reinforced stapling Suture Harmonic scalpel | 62 (80.5%) 30 (53.6%) 8 (10.4%) 7 (9.1%) | 10 (71.5%) 4 (50.0%) 1 (7.1%) 3 (21.4%) | 0.389 |
| Conversion to open surgery, n (%) | 1 (1.3%) | 0 (0%) | 0.668 |
| Clinically relevant postoperative pancreatic fistula (grade B/C), n (%) | 12 (16.2%) | 3 (21.4%) | 0.634 |
| Delayed gastric emptying, n (%) | 1 (1.3%) | 0 | 0.662 |
| Post-pancreatectomy hemorrhage, n (%) | 4 (5.4%) | 1 (7.1%) | 0.797 |
| Wound infection, n (%) | 0 | 0 | |
| Abdominal collection, n (%) | 11 (14.9%) | 3 (21.4%) | 0.538 |
| Sepsis, n (%) | 1 (1.3%) | 0 | 0.662 |
| Severe postoperative complications (Clavien-Dindo ≥ 3), n (%)[14] | 6 (7.8%) | 2 (14.3%) | 0.430 |
| Reintervention, n (%) | 3 (4.1%) | 1 (7.1%) | 0.620 |
| Readmission, n (%) | 6 (8.2%) | 0 | 0.266 |
| Median length of hospital stay [IQR], days | 7 [5-10] | 7.5 [6-15] | 0.193 |
| Postoperative mortality, n (%) | 0 | 0 |
DISCUSSION
In this first IGoMIPS analysis on minimally invasive distal pancreatectomy, we report on 110 SPDP and 578 DPWS. Our results are unique as they refer to present-day procedures (2019-2022) performed at all Italian centers participating in the Registry. In addition, IGoMIPS prospectively captures data on planned and performed procedures, allowing us to indicate when and how the spleen was preserved.
When compared to DPWS, patients undergoing SPDP were younger and the most frequent indication was neuroendocrine tumors at an early stage. From a technical point of view, DPWS entailed a higher risk of conversion to open surgery (7.6% vs. 0.9%) and SPDP was associated with a higher probability of deviation from the planned procedure (25% vs. 7.2%). Kimura SPDP (84.7%) was prevalent over Warshaw SPDP. The use of robotic assistance was prevalent in SPDP; however, the rate of spleen preservation was not increased when compared to laparoscopic SPDP. Outcomes of SPDP and DPWS were similar, but spleen preservation was associated with a trend toward fewer postoperative infections.
It has been sustained that post-splenectomy infective complications have an age-related impact due to the increased time-at-risk in the case of younger population and a possibly impaired function related to senescence of immune system in the case of the elderly population[15-21]. In our cohort, we did observe a lower median age in the case of patients who underwent SPDP. This could be related to patient selection (spleen preservation has been indicated more frequently in younger patients)[22] and to the epidemiology of benign/borderline malignant pancreatic tumors that are diagnosed more frequently in younger patients[23-25].
In our study, we observed a trend toward a lower rate of postoperative infections when the spleen was preserved (0.9% vs. 5.2%; P = 0.056). Similar results have previously been reported in the literature, although the pathophysiological mechanisms of increased early postoperative infectious complications after splenectomy are not yet understood[5,8,26].
Some authors have reported a reduction of intraoperative blood loss in SPDP compared to DPWS[3,7,8]. This finding is partially confirmed in this study due to the clinically minimal difference observed between the two groups. However, it should be interpreted in the light of smaller neuroendocrine tumors with limited retroperitoneal dissection, both of which could contribute to lower blood loss in SPDP.
Spleen preservation may also reduce the incidence of some postoperative complications, such as pancreatic fistula formation and delayed gastric emptying;[6,8,22,26-28] however, we did not observe this in our study. When comparing SPDP to DPWS, we did not find significant differences in postoperative outcome measures such as clinically relevant postoperative pancreatic fistula, post pancreatectomy hemorrhage, wound infections, abdominal fluid collections, need for hospital readmission, need for repeat surgery, length of hospital stay, and 90-day mortality.
Conversion to open surgery is a main outcome metric in minimally invasive surgery. Urgent conversion is associated with worse postoperative outcomes[29]. DPWS was associated with the increased need for conversion to open surgery (7.6% vs. 0.9%; P = 0.008). Again, patient selection and the extent of the procedure may be among the reasons explaining the lower conversion rate in SPDP. DPWS is frequently performed for pancreatic cancer, and in these patients, it is rather a radical antegrade modular pancreato-splenectomy. However, we confirmed that DPWS was associated with an increased risk of conversion to open surgery even when patients diagnosed with pancreatic cancer were excluded (5% vs. 0.9%; P = 0.045). Center and surgeon experience could account for some open conversion in DPWS. In this study, DPWS was performed at all centers, while SPDP was reported only by twenty-one centers. Some of these centers have an overall experience with thousands of pancreatic resections and report > 50 minimally invasive pancreatic resections per year to IGOMIPS[5].
A unique feature of this registry analysis was the possibility to define concordance between planned and performed procedures. In detail, splenectomy was required in 19% of the patients scheduled for SPDP. This is why some authors considered DPWS a “conversion procedure” when spleen preservation is not possible or in the presence of intraoperative complications[3].
In five patients, the final histology showed overtly malignant pancreatic tumors that would have required DPWS. It has been reported that when splenic vessels are resected en bloc, spleen preservation per se does not compromise oncological outcomes[30], most probably because the incidence of lymph node metastasis in station number 10 is exceedingly rare, even in pancreatic cancer[31]. The key oncological issue is that SPDP does not follow radical dissection planes and that preservation of splenic vessels is expected to increase the risk of margin positive resection and decreased lymph node yields. Preoperative selection is key to reducing the incidence of malignant histology following SPDP[32] and pancreatic surgeons should be aware of this risk.
Recent literature reports the potential advantages of the robotic platform over conventional laparoscopy for SPDP[33-37]. In accordance with these data, procedures enrolled in IGoMIPS demonstrated that robotic assistance was planned more frequently for SPDP than DPWS (50.9% vs. 29.7%; P < 0.001). However, robotic assistance and conventional laparoscopy were associated with similar rates of unplanned splenectomy (robotic 18.5% vs. laparoscopic 22.7%; P = 0.572). In addition, no difference was observed in the main postoperative outcome metrics. The lack of difference between robotic and laparoscopic SPDP could be explained by insufficient sample size. It could also be influenced by surgeon and center experience and the fact that not all centers had access to a robot platform. Indeed, we showed that two-thirds of SPDP were reported by just six centers, which reported the largest number of procedures per year.
A major technical controversy in SPDP is whether or not preservation of the splenic vessels is required (Kimura procedure) or could be omitted (Warshaw procedure). Undoubtedly, the Warshaw procedure is less demanding from a technical point of view. The controversy revolves around the possibility that resection of splenic vessels can increase the rate of early splenic complications, which may lead to clinically relevant left portal hypertension in the long term[38-40]. Longer follow-up is needed to determine the long-term consequences. Regarding postoperative outcomes, we could not define any relevant difference between Kimura and Warshaw procedures, but we underscore that the use of the Kimura procedure is more prevalent, as only fourteen Warshaw procedures were reported to IGoMIPS.
This study has several limitations. First, a limited number of centers reported the majority of procedures. Second, as in any multicenter study, interpretation of some postoperative complications could be influenced by subjective factors and accuracy of reporting and follow-up. Third, the study is a prospective, observational, non-randomized study. Fourth, robotic assistance is not available at all centers and may not be freely available even at a hospital with one or more robots. Fifth, although data on the variation of planned surgery are included in the registry, a dedicated query specifying the reason for performing a Warshaw procedure over a Kimura is not included. Despite these limitations, this study has several major strengths, such as the possibility of defining compliance with scheduled procedures and reporting on present-day operations on a prospective basis, giving an insight into the diffusion and short term-outcomes of minimally invasive pancreatic surgery in Italy. Even though the official number of Italian centers performing minimally invasive pancreatic resections is not available in literature, to the best of our knowledge, all the centers that have previously reported records of MIPS in literature are participating in the registry, giving the reader an idea of how representative the registry is of the Italian panorama.
SPDP appears to be feasible and safe if performed in centers with expertise in minimally invasive surgery. A widespread diffusion is still limited by technical aspects and limited indications. High-level evidence confirming the theoretical advantages provided by spleen preservation is still missing, but it appears to have outcomes not inferior to DPWS. Kimura procedure provides similar short-term outcomes to Warshaw procedure, reducing the risk of gastric infarction and gastric varices development. In experienced hands, both conventional laparoscopy and robot-assisted laparoscopy provide similar outcomes despite the theoretical advantages offered by the robot platform in providing a steady platform to dissect splenic vessels from the pancreas.
DECLARATIONS
AknowledgmentsTo Dr Valentina Giatti for the precious work of managing the database and to Professor Gianluca Baiocchi and RicerChiAmo Foundation for the generous support to the registry. To Associazione Italiana Studio Pancreas (AISP) for the promotion of and support for the registry.
Authors’ contributionMade substantial contributions to the conception and design of the study and performed data analysis and interpretation: Donisi G, Capretti G, Boggi U, Zerbi A
Performed data acquisition and provided administrative, technical, and material support: Donisi G, Capretti G, Napoli N, Partelli S, Esposito A, Ferrari G, Butturini G, Morelli L, Hilal MA, Viola M, Benedetto FD, Troisi R, Vivarelli M, Jovine E, Caputo D, Ferrero A, Bracale U, Alfieri S, Casadei R, Ercolani G, Moraldi L, Molino C, Valle RD, Ettorre G, Memeo R, Zanus G, Belli A, Gruttadauria S, Brolese A, Coratti A, Garulli G, Romagnoli R, Massani M, Belli G, Falconi M, Salvia R, Boggi U, Zerbi A
Availability of data and materialsData cannot be shared because they are extracted from the Italian National Registry of Minimally Invasive Pancreatic Surgery.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateAll centers participating in the registry received approval from their local Ethical Committee. Each participating patient has signed informed consent. The authorization number of the Ethical approval of Humanitas Institute is 2167.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Asbun HJ, Moekotte AL, Vissers FL, et al. The Miami international evidence-based guidelines on minimally invasive pancreas resection. Ann Surg 2020;271:1-14.
2. van Hilst J, de Rooij T, Klompmaker S, et al. European consortium on minimally invasive pancreatic surgery (E-MIPS). minimally invasive versus open distal pancreatectomy for ductal adenocarcinoma (DIPLOMA): a pan-european propensity score matched study. Ann Surg 2019;269:10-7.
3. Nakata K, Shikata S, Ohtsuka T, et al. Minimally invasive preservation versus splenectomy during distal pancreatectomy: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci 2018;25:476-88.
4. Moekotte AL, Lof S, White SA, et al. Minimally invasive liver and pancreatic surgery study group-UK (MI-LAPS UK). splenic preservation versus splenectomy in laparoscopic distal pancreatectomy: a propensity score-matched study. Surg Endosc 2020;34:1301-9.
5. Lee W, Hwang DW, Han HS, et al. Comparison of infectious complications after spleen preservation versus splenectomy during laparoscopic distal pancreatectomy for benign or low-grade malignant pancreatic tumors: a multicenter, propensity score-matched analysis. J Hepatobiliary Pancreat Sci 2023;30:252-62.
6. Choi SH, Seo MA, Hwang HK, Kang CM, Lee WJ. Is it worthwhile to preserve adult spleen in laparoscopic distal pancreatectomy? Perioperative and patient-reported outcome analysis. Surg Endosc 2012;26:3149-56.
7. Dai MH, Shi N, Xing C, et al. Splenic preservation in laparoscopic distal pancreatectomy. Br J Surg 2017;104:452-62.
8. Shi N, Liu SL, Li YT, You L, Dai MH, Zhao YP. Splenic preservation versus splenectomy during distal pancreatectomy: a systematic review and meta-analysis. Ann Surg Oncol 2016;23:365-74.
9. Kimura W, Yano M, Sugawara S, et al. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein: techniques and its significance. J Hepatobiliary Pancreat Sci 2010;17:813-23.
11. Zerbi A, Capretti G, Napoli N, et al. The italian national registry for minimally invasive pancreatic surgery: an initiative of the italian group of minimally invasive pancreas surgery (IGoMIPS). Updates Surg 2020;72:379-85.
12. Capretti G, Boggi U, Salvia R, et al. Application of minimally invasive pancreatic surgery: an Italian survey. Updates Surg 2019;71:97-103.
13. Bassi C, Marchegiani G, Dervenis C, et al. International study group on pancreatic surgery (ISGPS). the 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161:584-91.
14. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13.
15. Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risks. Br J Surg 1991;78:1031-8.
16. Chong J, Jones P, Spelman D, Leder K, Cheng AC. Overwhelming post-splenectomy sepsis in patients with asplenia and hyposplenia: a retrospective cohort study. Epidemiol Infect 2017;145:397-400.
17. Long B, Koyfman A, Gottlieb M. Complications in the adult asplenic patient: a review for the emergency clinician. Am J Emerg Med 2021;44:452-7.
18. Buzelé R, Barbier L, Sauvanet A, Fantin B. Medical complications following splenectomy. J Visc Surg 2016;153:277-86.
19. Hassenpflug M, Tjaden C, Hinz U, et al. Hypercoagulability after distal pancreatectomy: Just meaningless alterations? Pancreatology 2017;17:478-83.
20. Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood 2009;114:2861-8.
21. Turner VM, Mabbott NA. Influence of ageing on the microarchitecture of the spleen and lymph nodes. Biogerontology 2017;18:723-38.
22. Panda N, Bansal NK, Narsimhan M, Ardhanari R, Bronson JR. Spleen-preserving versus spleen-sacrificing distal pancreatectomy in laparoscopy and open method-perioperative outcome analysis-14 years experience. Indian J Surg 2016;78:90-5.
23. Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727-33.
24. Dababneh Y, Mousa OY. Pancreatic serous cystadenoma. https://www.ncbi.nlm.nih.gov/books/NBK557432/ [Last accessed on 22 Mar 2023] .
25. Babiker HM, Hoilat GJ, Mukkamalla SKR. Mucinous cystic pancreatic neoplasms. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448105/ [Last accessed on 22 Mar 2023].
26. Pendola F, Gadde R, Ripat C, et al. Distal pancreatectomy for benign and low grade malignant tumors: short-term postoperative outcomes of spleen preservation-a systematic review and update meta-analysis. J Surg Oncol 2017;115:137-43.
27. Kwon W, Jang JY, Kim JH, et al. An analysis of complications, quality of life, and nutritional index after laparoscopic distal pancreatectomy with regard to spleen preservation. J Laparoendosc Adv Surg Tech A 2016;26:335-42.
28. Zhao YP, Du X, Dai MH, et al. Laparoscopic distal pancreatectomy with or without splenectomy: spleen-preservation does not increase morbidity. Hepatobiliary Pancreat Dis Int 2012;11:536-41.
29. Lof S, Korrel M, van Hilst J, et al. Outcomes of elective and emergency conversion in minimally invasive distal pancreatectomy for pancreatic ductal adenocarcinoma: an international multicenter propensity score-matched study. Ann Surg 2021;274:e1001-7.
30. Kawaguchi Y, Fuks D, Nomi T, Levard H, Gayet B. Laparoscopic distal pancreatectomy employing radical en bloc procedure for adenocarcinoma: technical details and outcomes. Surgery 2015;157:1106-12.
31. Seo S, Uemura K, Sumiyoshi T, et al. Optimal lymph-node dissection for pancreatic tail cancer. Surg Today 2022;52:1307-12.
32. Ramia JM, Del Rio Martín J, Blanco-Fernández G, et al. Pancreatic mucinous cystic neoplasms located in the distal pancreas: a multicenter study. Gland Surg 2022;11:795-804.
33. Chen S, Zhan Q, Chen JZ, et al. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc 2015;29:3507-18.
34. Chen P, Zhou B, Wang T, Hu X, Ye Y, Guo W. Comparative efficacy of robot-assisted and laparoscopic distal pancreatectomy: a single-center comparative study. J Healthc Eng 2022;2022:7302222.
35. Kang CM, Kim DH, Lee WJ, Chi HS. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9.
36. Yang SJ, Hwang HK, Kang CM, Lee WJ. Revisiting the potential advantage of robotic surgical system in spleen-preserving distal pancreatectomy over conventional laparoscopic approach. Ann Transl Med 2020;8:188.
37. Eckhardt S, Schicker C, Maurer E, Fendrich V, Bartsch DK. Robotic-assisted approach improves vessel preservation in spleen-preserving distal pancreatectomy. Dig Surg 2016;33:406-13.
38. Hang K, Zhou L, Liu H, et al. Splenic vessels preserving versus Warshaw technique in spleen preserving distal pancreatectomy: a systematic review and meta-analysis. Int J Surg 2022;103:106686.
39. Lee LS, Hwang HK, Kang CM, Lee WJ. Minimally invasive approach for spleen-preserving distal pancreatectomy: a comparative analysis of postoperative complication between splenic vessel conserving and Warshaw's technique. J Gastrointest Surg 2016;20:1464-70.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Donisi G, Capretti G, Napoli N, Partelli S, Esposito A, Ferrari G, Butturini G, Morelli L, Hilal MA, Viola M, Benedetto FD, Troisi R, Vivarelli M, Jovine E, Caputo D, Ferrero A, Bracale U, Alfieri S, Casadei R, Ercolani G, Moraldi L, Molino C, Valle RD, Ettorre G, Memeo R, Zanus G, Belli A, Gruttadauria S, Brolese A, Coratti A, Garulli G, Romagnoli R, Massani M, Belli G, Falconi M, Salvia R, Boggi U, Zerbi A. Minimally invasive spleen-preserving distal pancreatectomy: real-world data from the Italian National Registry of Minimally Invasive Pancreatic Surgery (IGoMIPS). Mini-invasive Surg 2023;7:7. http://dx.doi.org/10.20517/2574-1225.2022.92
AMA Style
Donisi G, Capretti G, Napoli N, Partelli S, Esposito A, Ferrari G, Butturini G, Morelli L, Hilal MA, Viola M, Benedetto FD, Troisi R, Vivarelli M, Jovine E, Caputo D, Ferrero A, Bracale U, Alfieri S, Casadei R, Ercolani G, Moraldi L, Molino C, Valle RD, Ettorre G, Memeo R, Zanus G, Belli A, Gruttadauria S, Brolese A, Coratti A, Garulli G, Romagnoli R, Massani M, Belli G, Falconi M, Salvia R, Boggi U, Zerbi A. Minimally invasive spleen-preserving distal pancreatectomy: real-world data from the Italian National Registry of Minimally Invasive Pancreatic Surgery (IGoMIPS). Mini-invasive Surgery. 2023; 7: 7. http://dx.doi.org/10.20517/2574-1225.2022.92
Chicago/Turabian Style
Donisi, Greta, Giovanni Capretti, Niccolò Napoli, Stefano Partelli, Alessandro Esposito, Giovanni Ferrari, Giovanni Butturini, Luca Morelli, Mohammad Abu Hilal, Massimo Viola, Fabrizio Di Benedetto, Roberto Troisi, Marco Vivarelli, Elio Jovine, Damiano Caputo, Alessandro Ferrero, Umberto Bracale, Sergio Alfieri, Riccardo Casadei, Giorgio Ercolani, Luca Moraldi, Carlo Molino, Raffaele Dalla Valle, Giuseppe Ettorre, Riccardo Memeo, Giacomo Zanus, Andrea Belli, Salvatore Gruttadauria, Alberto Brolese, Andrea Coratti, Gianluca Garulli, Renato Romagnoli, Marco Massani, Giulio Belli, Massimo Falconi, Roberto Salvia, Ugo Boggi, Alessandro Zerbi. 2023. "Minimally invasive spleen-preserving distal pancreatectomy: real-world data from the Italian National Registry of Minimally Invasive Pancreatic Surgery (IGoMIPS)" Mini-invasive Surgery. 7: 7. http://dx.doi.org/10.20517/2574-1225.2022.92
ACS Style
Donisi, G.; Capretti G.; Napoli N.; Partelli S.; Esposito A.; Ferrari G.; Butturini G.; Morelli L.; Hilal MA.; Viola M.; Benedetto FD.; Troisi R.; Vivarelli M.; Jovine E.; Caputo D.; Ferrero A.; Bracale U.; Alfieri S.; Casadei R.; Ercolani G.; Moraldi L.; Molino C.; Valle RD.; Ettorre G.; Memeo R.; Zanus G.; Belli A.; Gruttadauria S.; Brolese A.; Coratti A.; Garulli G.; Romagnoli R.; Massani M.; Belli G.; Falconi M.; Salvia R.; Boggi U.; Zerbi A. Minimally invasive spleen-preserving distal pancreatectomy: real-world data from the Italian National Registry of Minimally Invasive Pancreatic Surgery (IGoMIPS). Mini-invasive. Surg. 2023, 7, 7. http://dx.doi.org/10.20517/2574-1225.2022.92
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 12 clicks
Cite This Article 12 clicks











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.