Minimally invasive isolated anatomic liver segmentectomy for hepatocellular carcinoma using extrahepatic Glissonian approach: surgical techniques and outcomes
Abstract
Aim: To standardize surgical techniques for and define the safety, feasibility and oncologic validity of minimally invasive anatomic liver segmentectomy for hepatocellular carcinoma (HCC).
Methods: We retrospectively studied perioperative and long-term outcomes of isolated anatomic segmentectomy (IA-Seg) using the extrahepatic Glissonian approach in 157 HCC cases, including 77 open and 80 minimally invasive (59 laparoscopic and 21 robotic) cases. Surgical outcomes were compared between the approaches using propensity score matching (PSM).
Results: After matching (46:46), compared with open IA-Seg, minimally invasive IA-Seg was significantly associated with less blood loss (274 vs. 955 g), a lower transfusion rate (21.7% vs. 45.7%), the lower postoperative serum total bilirubin (TB) level (1.5 vs. 2.2 mg/dL) and shorter length of hospital stay (LOS) (17 vs. 27 days), while the latter had a significantly higher rate of Pringle maneuver application (15.2% vs. 2.2%) and a higher aspartate aminotransferase (AST) level (669 vs. 402 IU/L). Additionally, laparoscopic and robotic IA-Seg before and after matching (16:16) had comparable perioperative outcomes. Long-term outcomes after IA-Seg for newly developed HCC in matched cohorts were comparable, either between open and minimally invasive IA-Seg (36:36) or between laparoscopic and robotic IA-Seg (12:12).
Conclusion: Although minimally invasive IA-Seg is technically demanding, it could be standardized using the extrahepatic Glissonian approach. This procedure for HCC was safe, feasible and oncologically acceptable, with several perioperative outcomes superior to those in open IA-Seg and with comparable long-term outcomes. By expert hands, the laparoscopic or robotic approach could be a reliable option for IA-Seg in selected HCC patients.
Keywords
INTRODUCTION
Isolated anatomic (sub)segmentectomy (IA-Seg) is a hepatectomy procedure, where an anatomically determined territory that is supplied by the third- (or fourth-) order division Glissonian or portal pedicles (GPs) is completely and optimally resected. IA-Seg is a type of parenchyma-preserving anatomic hepatectomy that can be applied to attain both high curability and functional safety in liver resection for malignancy. Therefore, IA-Seg is regarded to confer benefits to surgical management of hepatocellular carcinoma (HCC), which is characterized by intra-portal vein tumor spread and accompanying impaired hepatic functional reserve[1-3].
Accurate IA-Seg, particularly for posterosuperior liver segments, is complex and remains technically challenging, either in the open, laparoscopic or robotic setting. In addition, surgical techniques of IA-Seg are not standardized in minimally invasive surgery (MIS) or even in open surgery[1,4-10]. In particular, the methods to determine the anatomically isolated segments to be resected are not established in MIS. For IA-Seg, two principal methods to determine the anatomically isolated liver segments have been proposed[1,6,8-12]. One is the “segment-staining method”, where dye is directly injected into the portal vein branches that supply the target segments by manual needle punctures under ultrasound (US) guidance[1,6,11]. This method devised by Makuuchi is widely used in open surgery[1], but it poses significant difficulties or needs special expertise in MIS because of technical or instrumental insufficiency[11]. The other is the conventional “negative-coloring method” without the use of dye or the “negative-staining method” which visualizes the target segment as a negatively stained area through systemic injection of indocyanine green (ICG), after occluding the target segmental GPs that are isolated intrahepatically or extrahepatically[8-10,12-15]. Recent technical refinements in isolation of segmental GPs, as well as advances in fluorescence navigation technology, have promoted the application of the negative-staining method in open and minimally invasive IA-Seg.
A literature review found that no previous study has compared the surgical outcomes between open and minimally invasive IA-Seg for HCC covering a variety of liver segments. In this single-center study on the 157 consecutive IA-Seg cases of HCC, including 77 open surgery and 80 MIS cases, we present our standardized surgical techniques of IA-Seg and compare perioperative and long-term outcomes between open and minimally invasive IA-Seg, using propensity score matching (PSM) analyses. Additionally, to examine the potential impact of robotics on minimally invasive IA-Seg, we further compare surgical outcomes between 59 laparoscopic and 21 robotic IA-Seg cases.
METHODS
Surgical indications for open or minimally invasive IA-Seg
At our institution, IA-Seg was the first choice of liver resection procedure for small or medium-sized HCCs, if they were confined to one liver segment and IA-Seg was oncologically and technically appropriate, depending on the tumor location, tumor macroscopic form, potential vascular invasion, patients’ hepatic functional reserve and their physical status. Surgical indication of IA-Seg was also determined mostly according to the so-called Makuuchi criteria[16]. In cases where tumors were located close to or across the border of the adjacent liver segment, and in cases where actual or potential vascular invasion in the adjacent segment was suspected, extended IA-Seg or combined resection of part of the contiguous segment was performed. Furthermore, IA-Seg was performed even for large tumors, if the future remnant liver volume was small or the patient’s hepatic reserve was functionally unbearable for more extensive procedures. Notably, IA-Seg was not conducted in cases where sectionectomy or even hemihepatectomy is oncologically or functionally appropriate. Furthermore, non-anatomic resection (NAR) was selected rather than IA-Seg in selected repeat hepatectomy cases, if NAR was oncologically acceptable depending on the tumor characteristics, and when it was aimed to preserve the liver parenchyma volume to prepare for the future treatment of potential intrahepatic recurrence.
Selection of open or minimally invasive IA-Seg was dependent on the era, tumor size, tumor location and surgeons’ capability. Minimally invasive IA-Seg was basically indicated for the following conditions: (1) tumors with a diameter ≤ 15 cm, without limitation of tumor location; (2) five or fewer excision sites; and (3) hepatectomy without requiring biliary or vascular reconstruction.
Surgeries were performed by all of the authors with the expertise of both open and minimally invasive IA-Seg. Until 2015 before the start of national insurance coverage of minimally invasive IA-Seg in Japan, the open approach was the first choice for IA-Seg, while from 2016, the laparoscopic approach had the priority when indicated. Until March 2022, robotic liver resection was a practice at patients’ own expense in Japan, which significantly affected the selection between the MIS approaches. Since April 2022, the robotic approach has been covered by national insurance and has become the first choice for IA-Seg at our institution.
Background data collection
Patient and tumor background data were retrospectively collected from their medical charts. The data included age, sex, body mass index (BMI), American Society of Anesthesiology (ASA)- Performance Status score, Child-Pugh grade, serum biomarkers, ICG retention rate at 15 min (ICGR15), histologically proven cirrhosis (postoperative evaluation), etiology of background liver disease (viral or non-viral) and previous hepatectomy. The number, size and location of the tumors and preoperative serum tumor markers [alpha-fetoprotein (AFP); Des-gamma-carboxy prothrombin (DCP)] were examined. Posterosuperior segments were defined as segments Sg1, Sg4a, Sg7 and Sg8, and the others were classified as anterolateral segments. Pathological tumor stages[17] and differentiation grades were determined.
Terminology and definition of IA-Seg
The terminology for liver anatomy and procedures of hepatectomy was primarily based on the Brisbane 2000 Terminology of Liver Anatomy and Resections[18] and Couinaud’s classification[19]. IA-Seg was defined as a type of procedure to resect an isolated liver territory that is supplied by the third- or fourth-order division GPs or by its combination with the adjacent GPs smaller than the sectional GPs. Thus, IA-Seg included mono-segmentectomy (the third-order division), subsegmentectomy (the fourth-order division) and their combinations, less than a sectionectomy. Isolated total caudate lobectomy was defined as mono-segmentectomy, and complete resection of the Spiegel lobe by dividing the left caudate GPs at its root was defined as subsegmentectomy.
Evaluation of liver segmental anatomy
Liver segmental anatomy including the third- or fourth-order division GPs and the major or intersegmental hepatic veins were preoperatively evaluated using axial CT and MRI imaging, as well as a 3-D reconstruction software (Synapse Vincent ®, Fujifilm, Japan).
Surgical techniques for IA-Seg
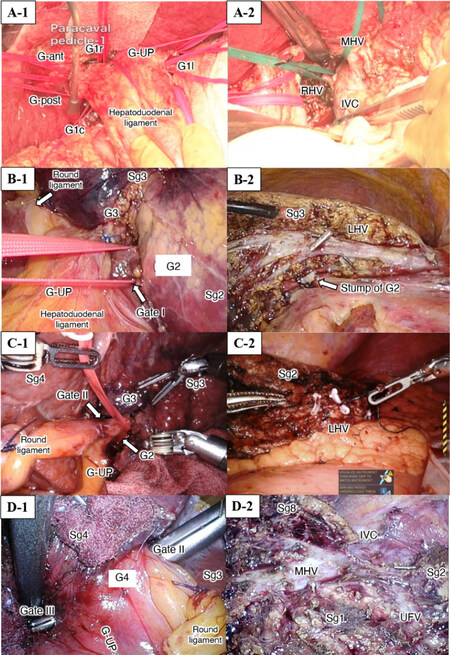
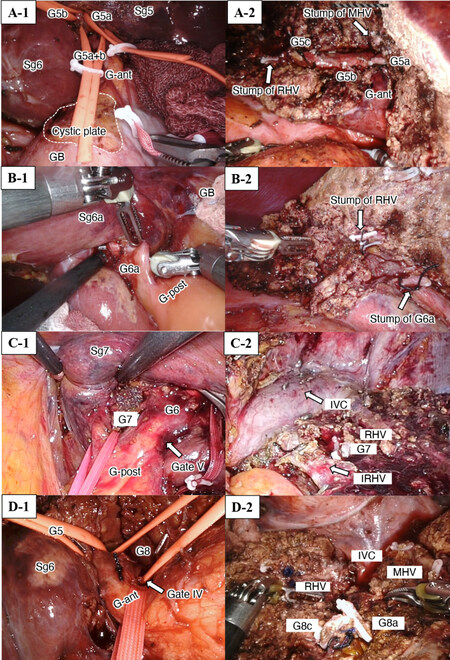
Surgical techniques for IA-Seg were based on extrahepatic isolation and clamping of the target segmental GPs (the extrahepatic Glissonian approach), followed by dissection of the optimal amount of parenchyma that was determined along the demarcation line, with exposure of landmark hepatic veins, when applicable. These techniques were based on the anatomical background of Laennec’s capsule at the hilum and major hepatic veins and described elsewhere[9,12,13,15,20]. Figures 1 and 2 show the extrahepatic isolation of the target segmental GPs in all segments from Sg1 to Sg8, as well as the exposure or division of the landmark hepatic veins after resection of the corresponding segments in open or minimally invasive IA-Seg.
Figure 1. Extrahepatic isolation of the segmental Glissonian pedicles (A to D-1) and exposure of the landmark hepatic veins (A to D-2) during IA-Seg (Sg1, Sg2, Sg3, Sg4). (A) Open isolated caudate lobectomy; (B) laparoscopic segmentectomy 2; (C) robotic segmentectomy 3; (D) laparoscopic segmentectomy 4. G-ant and G-post: Glissonian pedicles of the anterior and posterior sections, respectively; G-UP: left Glissonian pedicle at the root of the umbilical portion of the portal vein; GB: gallgladder; IVC: inferior vena cava; LHV: left hepatic vein; MHV: middle hepatic vein; UFV: umbilical fissure vein.
Figure 2. Extrahepatic isolation of the segmental Glissonian pedicles (A to D-1) and exposure or division of the landmark hepatic veins (A to D-2) during IA-Seg (Sg5, Sg6a, Sg7, Sg8). (A) Robotic subsegmentectomy S5; (B) robotic subsegmentectomy 6; (C) robotic segmentectomy 7; (D) robotic segmentectomy 8. G-ant and G-post: Glissonian pedicles of the anterior and posterior sections, respectively; GB: gall bladder; IVC: inferior vena cava; MHV: middle hepatic vein; RHV: right hepatic vein.
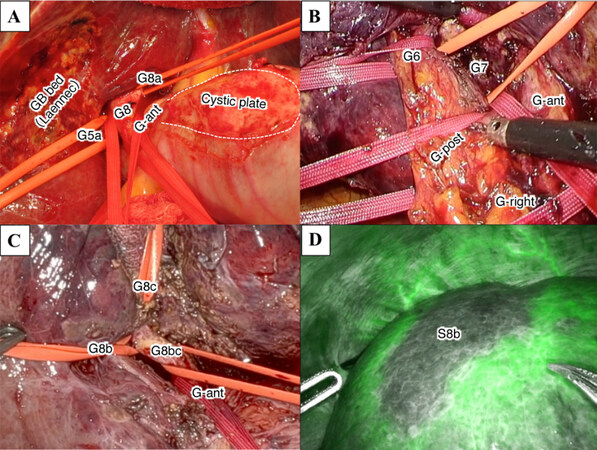
Isolation of the target (sug)segmental GPs in the right-side (sub)segmentectomies such as segmentectomy 5, 6, 7 and 8 was shown in further detail in Figure 3. To isolate these pedicles, we usually isolated the more proximal second-order division GP [the right anterior(G-ant) or posterior(G-post) sectional GP] extrahepatically at first, followed by isolation of the third- or fourth-order division GP that branched off the sectional GPs [Figure 3A, B and C]. During this procedure, by retracting the looped second-order division (sectional) GP in the caudo-dorsal direction, we identified the third-order division GPs and looped them. Further, we isolated the fourth-order division (subsegmental) GPs after isolating the third-order division (segmental) GPs which the subsegmental GPs rooted. To isolate deep (sub)segmental GPs in cases of (sub)segmentectomy 7 or 8, we also often used ‘the subtraction method’, where the one end of the tape with which we looped the proximal sectional GP was passed under the more superficial segmental GP in front of the deeper GP [Figure 3A, B and C]. Using this technique, we indirectly isolated the deep (sub)segmental GPs. Additionally, to isolate the segmental pedicles in the left-side segmentectomies such as segment (Sg) 2, 3 and 4 pedicles, we were able to directly isolate these third-order division GPs extrahepatically
Figure 3. Extrahepatic Glissonian approach for anatomic (sub)segmentectomy using the subtraction method. (A) Extrahepatic isolation of Glissonian pedicles to Sg8 (G8), anterior Sg5 (G5a) and Sg8 (G8a) subsegments and anterior section (G-ant) during open anatomic subsegmentectomy 8a. Laennec’s capsule on the gall bladder (GB) bed and the cystic plate (area inside the dotted line) resected along with GB are shown; (B) extrahepatic isolation of Glissonian pedicles to Sg6 (G6), Sg7 (G7), anterior (G-ant) and posterior (G-post) sections and right hemiliver (G-right) during laparoscopic anatomic segmentectomy 7; (C) extrahepatic isolation of Glissonian pedicles to lateral (G8b) and dorsal (G8c) subsegments of Sg8, their root (G8bc) and G-ant during robotic anatomic subsegmentectomy Sg8b; (D) [same case with (C)] Isolated Sg8b ischemia shown using “negative staining technique” in the Firefly mode after systemic infusion of ICG during selective clamping of G8b.
In isolated caudate lobectomy (segmentectomy 1), we applied two approaches[21]. One was the central hilar approach, and the other was “the left-to-right tracking technique” or “the left-side approach”. Briefly, in the former approach [Figure 1A-1], hepatoduodenal ligament (HDL), G-ant, G-post, left Glissonian pedicle at the root of the umbilical portion of the portal vein (G-UP), and Arantius plate (AP) were isolated extrahepatically at first. Then, pedicles of the left caudate lobe (Spiegel lobe) (G1l) and the caudate process (G1c) are directly isolated extrahepatically. Finally, by repeating the subtraction method, the tape with which HDL was looped was passed under all of G1c, G-post, G-ant, AP, and G-UP, and finally, we indirectly isolated pedicles of the paracaval portion of the caudate lobe (G1r). In the latter approach (the left-side approach), all caudate pedicles (G1l, G1c, G1r) were directly isolated extrahepatically and divided one by one from the left side. At first, G1l was isolated and divided. From the left side view, by keeping the dissection layer on the hilar plate, several G1r and G1c were isolated extrahepatically at their origins from the hilar plate and divided one by one from the left side.
Division of the isolated target (sub)segmental GPs caused the selective ischemia of the isolated (sub)segments to be resected. The ischemic area was confirmed macroscopically as a discolored area, ultrasonically, or by using ICG negative staining technique, either in the open, laparoscopic or robotic (Firefly mode) setting [Figure 3D].
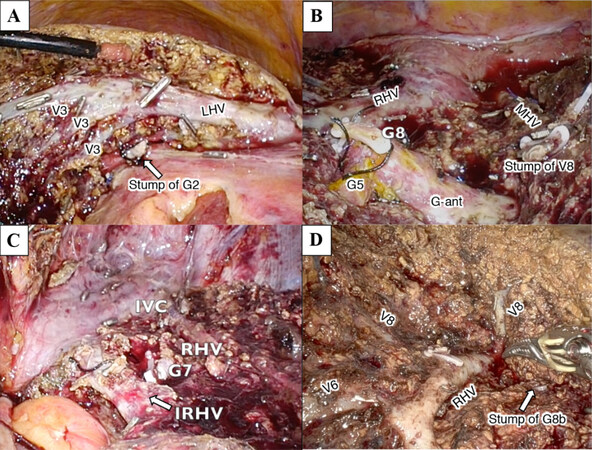
After selective occlusion of the inflow to the anatomic (sub)segments to be resected, parenchymal dissection was started on the demarcation line. In cases where the landmark major hepatic vein lied on the border between the target and adjacent segments, the landmark vein was exposed from its root side to the peripheral side [hepatic vein (HV) root-at first parenchymal dissection]. During parenchymal dissection, the target GPs that had been clamped in the hilar procedure were exposed on the liver cutting plane, and they were divided at this time. Parenchymal dissection proceeded in the cranial-to-caudal direction and IA-Seg was completed [Figures 1, 2 and 4].
Figure 4. Exposure of landmark hepatic veins after parenchymal dissection during anatomic (sug)segmentectomy. (A) Image after completion of laparoscopic anatomic segmentectomy 2. Left hepatic vein (LHV) and venous tributaries from Sg3 (V3) are exposed. The resected stump of Glissonian pedicle to Sg2 (G2, arrow) is shown beneath LHV; (B) image after completion of laparoscopic anatomic segmentectomy 8. The resected stump of G8, preserved G5 and G-ant, stump of V8 and the right (RHV) and middle (MHV) hepatic veins are exposed on the liver cutting surface; (C) image after completion of laparoscopic anatomic segmentectomy 7 in the left semi-prone patient position. Inferior vena cava (IVC), RHV, inferior RHV (IRHV, arrow) and the resected stump of G7 are exposed; (D) image after completion of robotic anatomic subsegmentectomy 8b. The resected stump of G8b (arrow), RHV and its venous tributaries (V8 and V6) are exposed on the liver cutting surface.
In this series, these surgical techniques of IA-Seg were consistently applied either in the open, laparoscopic or robotic approach. Further, these techniques were applicable to almost all types of IA-Seg, irrespective of the location of the target segment. For parenchymal dissection, Cavitron Ultrasonic Surgical Aspirator (CUSA ®, INTEGRA, USA) was used in open and laparoscopic cases, and the crush-clamping method and ultrasonic coagulating shears were used in robotic cases. As a robotic platform, da Vinci Surgical System ® (Intuitive Surgical, USA) was used in all cases. Our standardized methods of extrahepatic isolation of the target (sub)segmental GPs and parenchymal dissection are summarized in Figures 1 to 4, and were previously reported elsewhere[9,12,15,21]. The Pringle maneuver was not used routinely but applied on demand.
Perioperative data
Intraoperative outcomes were evaluated by operative time, blood loss, liver parenchymal dissection time, transfusion (of any blood elements), use of Pringle maneuver, open conversion (in minimally invasive IA-Seg), and operative difficulty according to the IWATE criteria[22].
Postoperative outcomes were evaluated by serum levels of maximum TB and aspartate aminotransferase (AST) and minimum platelet count (PC), complications graded according to the Clavien-Dindo (CD) classification[23], R0 resection, and the postoperative length of hospital stay (LOS). Overall and major complications were defined as those within 90 postoperative days of any CD grade and of ≥ grade IIIa, respectively.
Statistical analysis
Continuous data were expressed as median with range (background data) or interquartile range (perioperative data), and compared using the Kruskal-Wallis test. Categorical data were compared using the chi-square test or Fisher’s exact test, as appropriate. In some comparative studies, 1:1 PSM was conducted to reduce biases. In a comparison of perioperative outcomes between the open and MIS cohorts, the following nine variables were matched for PSM: age, sex, ASA class (I or II/≥ III), ICGR15 (< 13.0%/≥ 13.0%), tumor number (single/multiple), tumor size (< 3.0 cm/≥ 3.0 cm), tumor location (anterolateral/posterosuperior), tumor stage (I or II/≥ III), and previous hepatectomy (yes/no). In a comparison of perioperative outcomes between the laparoscopic and robotic cohorts, age, sex, cirrhosis (yes/no), tumor number (single/multiple), tumor size (< 3.0 cm/≥ 3.0 cm) and previous hepatectomy (yes/no) were matched. In a comparison of long-term outcomes between the open and MIS cohorts, age, sex, ASA class, ICGR15, tumor number, tumor size, tumor stages and tumor differentiation were matched. In a comparison of long-term outcomes between the laparoscopic and robotic cohorts, age, sex, tumor number, tumor size and presence of cirrhosis were matched.
The PSM method was the nearest neighborhood method with a caliper width of 0.20. The standard mean deviation (SMD) was calculated for all studied variables, and an SMD < 0.20 was confirmed for all matched variables, which indicated appropriate matching. The postoperative overall survival (OS) and recurrence-free survival (RFS) were analyzed only in patients with newly developed HCC using the Kaplan-Meier method. P < 0.050 was considered statistically significant. Statistical analyses were performed using JMP® software ver. 14.0 (SAS Institute, NC, USA).
The study design
The patients’ data were collected from their medical charts. The study was conducted under approval by the Institutional Regulation Board (approval number: HM19-064) and in accordance with the Declaration of Helsinki (2000). Informed consent was obtained as appropriate.
RESULTS
Between 2010 and June 2022, we performed 667 liver resections for HCC, including 306 open, 279 laparoscopic and 82 robotic resections, at our institution. According to the selection criteria described in the METHODS section, we excluded 229 open and 281 MIS cases from these 667 cases for this study. These 229 open cases included 121 cases of NAR, 5 cases of left lateral sectionectomy (LLS), 56 cases of sectionectomy, and 47 cases of bi- or tri-sectionectomy. The excluded 281 MIS cases consisted of 219 cases of NAR, 8 cases of LLS, 30 cases of sectionectomy, 21 cases of bi- or tri-sectionectomy, and 3 cases of IA-Seg in which the extrahepatic Glissonian approach was not applied.
As a result, we finally enrolled and retrospectively reviewed the 157 consecutive cases, consisting of 77 open and 80 MIS (59 laparoscopic and 21 robotic) cases, where IA-Seg was performed using the extrahepatic Glissonian approach. For robotic IA-Seg, we used the da Vinci Surgical System ® Si platform in 3 cases and the Xi platform in 18 cases.
The resected segments (including subsegments) and distribution (anterolateral or posterosuperior) were described in Table 1. As shown, the resection of posterosuperior segments accounted for 60.0% or more in both open and MIS groups.
Resected isolated liver segments
| Open (n = 77) | MIS (n = 80) | |||
| Laparoscopic (n = 59) | Robotic (n = 21) | Total | ||
| Resected Segment*, n | ||||
| Sg1 | 3 | 4 | 2 | 6 |
| Sg2 | 1 | 2 | 2 | 4 |
| Sg3 | 3 | 5 | 1 | 6 |
| Sg4 | 4 | 0 | 1 | 1 |
| Sg5 | 18 | 8 | 3 | 11 |
| Sg6 | 9 | 7 | 4 | 11 |
| Sg7 | 13 | 9 | 0 | 9 |
| Sg8 | 26 | 24 | 8 | 32 |
| Location | ||||
| Anterolateral, n (%) | 28 (36.4) | 21 (35.6) | 11 (52.4) | 32 (40.0) |
| Posterosuperior, n (%) | 49 (63.6) | 38 (64.4) | 10 (47.6) | 48 (60.0) |
Perioperative outcomes in IA-Seg for HCC
Comparison between the open and MIS approaches
Patient and tumor background data
Patient and tumor background data were compared between open surgery and MIS [Table 2]. Before PSM (77 open and 80 MIS cases), compared to open surgery, MIS was significantly associated with the lower ASA class, lower ICGR15, smaller tumor number and more favorable tumor stage. Further, MIS tended to be associated with higher BMI, the lower Child-Pugh class, small tumor size and lower DCP level, without statistical significance. After 1:1 PSM (46:46), the open and MIS groups were comparable in terms of all studied variables.
Comparison of background data between open and MIS cohorts undergoing segmentectomy for HCC before and after PSM
| Before PSM | After PSM | |||||
| Open (n = 77) | MIS (n = 80) (Lap 59, Robot 21) | P | Open (n = 46) | MIS (n = 46) (Lap 32, Robot 14) | P | |
| Age, years | 73 (43-91) | 71 (36-86) | 0.156 | 72 (43-91) | 72 (36-86) | 0.749 |
| Sex, male/female | 61/16 | 66/14 | 0.601 | 36/10 | 37/9 | 0.797 |
| BMI, kg/m2 | 22.6 (17.2-54.0) | 23.5 (17.9-36.3) | 0.076 | 23.1 (17.3-30.7) | 23.4 (18.0-33.9) | 0.437 |
| ASA score, I or II/≥ III | 57/20 | 77/3 | < 0.0001 | 44/2 | 44/2 | 1.000 |
| Child-Pugh class, A/B/C | 72/5/0 | 79/1/0 | 0.087 | 44/2/0 | 45/1/0 | 0.557 |
| Cirrhosis (pathologic), n (%) | 34 (44.2) | 33 (41.3) | 0.713 | 21 (45.7) | 21 (45.7) | 1.000 |
| ICGR15, % ≥ 13.0%, n (%) | 15.1 (0.6-41.0) 46 (61.3) | 11.2 (2.2-68.3) 29 (38.2) | 0.006 0.004 | 14.1 (0.6-41.0) 20 (43.5) | 14.3 (2.2-68.3) 18 (39.1) | 0.623 0.672 |
| Tumor number Single/Multiple | 1 (1-23) 52/25 | 1 (1-4) 66/14 | 0.034 0.030 | 1 (1-6) 35/11 | 1 (1-4) 36/10 | 0.898 0.804 |
| Tumor size, cm ≥ 3.0 cm, n (%) | 3.3 (1.2-17.0) 46 (59.7) | 3.0 (0.7-11.0) 40 (50.0) | 0.054 0.220 | 3.5 (1.2-17.0) 27 (58.7) | 3.1 (0.7-11.0) 25 (54.4) | 0.176 0.674 |
| Etiology, viral/non-viral | 52/25 | 49/31 | 0.411 | 28/18 | 27/19 | 0.832 |
| Anterolateral/Posterosuperior | 28/49 | 32/48 | 0.639 | 19/27 | 17/29 | 0.669 |
| AFP, ng/mL | 10.4 (2.2-28,180.0) | 6.1 (1.0-15,412.0) | 0.133 | 8.8 (2.3-9,439.5) | 8.7 (1.0-15,412.0) | 0.683 |
| DCP, mAU/mL | 95 (10-123,650) | 50 (10-30,899) | 0.076 | 107 (10-123,650) | 51 (10-30,899) | 0.263 |
| Pathological stage, I or II/≥ III | 49/28 | 67/13 | 0.004 | 35/11 | 35/11 | 1.000 |
| Previous hepatectomy Yes, n (%) Number, 0/1/≥ 2 Including open Hx, n (%) | 14 (18.2) 63/14/0 13 (13.9) | 15 (18.8) 65/12/3 10 (12.5) | 0.927 0.209 0.438 | 8 (17.4) 38/8/0 7 (15.2) | 10 (21.7) 37/6/3 6 (13.0) | 0.599 0.192 0.765 |
Perioperative outcomes
Perioperative outcomes are shown in Table 3. Before PSM, compared to the open approach (n = 77), MIS
Comparison of perioperative outcomes between open and MIS cohorts undergoing segmentectomy for HCC before and after PSM
| Before PSM | After PSM | |||||
| Open (n = 77) | MIS (n = 80) (Lap 59, Robot 21) | P | Open (n = 46) | MIS (n = 46) (Lap 32, Robot 14) | P | |
| Operative time, min | 570 (464-752) | 654 (529-828) | 0.055 | 555 (470-709) | 656 (536-833) | 0.059 |
| Blood loss, g | 988 (538-1,611) | 218 (119-544) | < 0.0001 | 955 (580-1,427) | 274 (132-709) | < 0.0001 |
| Transfusion, n (%) | 38 (49.4) | 14 (17.5) | < 0.0001 | 21 (45.7) | 10 (21.7) | 0.015 |
| Pringle maneuver, n (%) | 5 (6.5) | 14 (17.5) | 0.035 | 1 (2.2) | 7 (15.2) | 0.026 |
| Open conversion, n (%) | NA | 1 (1.3) | NA | NA | 0 (0) | NA |
| Max-TB, mg/dL | 2.2 (1.5-3.1) | 1.5 (1.3-2.0) | < 0.0001 | 2.2 (1.5-2.6) | 1.5 (1.3-1.9) | 0.0008 |
| Max-AST, IU/L | 399 (292-627) | 626 (341-1,103) | 0.001 | 402 (291-743) | 669 (407-1,204) | 0.008 |
| Min-PT, % | 63 (54-69) | 63 (57-72) | 0.444 | 63 (54-68) | 63 (58-70) | 0.453 |
| R0 resection, % | 94.8 | 100 | 0.039 | 95.7 | 100 | 0.153 |
| Morbidity ≤ 90 days, n (%) Overall Major (≥ C-D IIIa) Bile leak/collection | 46 (59.7) 12 (15.6) 7 (9.1) | 34 (42.5) 9 (11.3) 3 (3.8) | 0.031 0.425 0.171 | 28 (60.9) 8 (17.4) 5 (10.9) | 21 (45.7) 6 (13.0) 1 (2.2) | 0.144 0.562 0.091 |
| Mortality, n (%) ≤ 30 days ≤ 90 days | 1 (1.3) 3 (3.9) | 0 (0) 1 (1.3) | 0.307 0.293 | 1 (2.2) 2 (4.4) | 0 (0) 0 (0) | 0.315 0.153 |
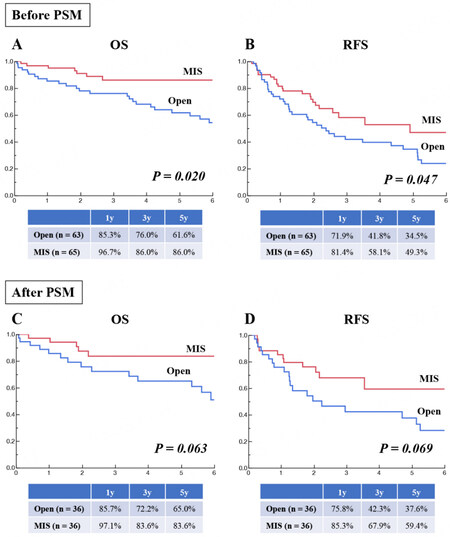
| Length of hospital stay, days | 29 (21-38) | 15 (13-20) | < 0.0001 | 27 (19-33) | 17 (13-23) | < 0.0001 |
Comparison between the laparoscopic and robotic approaches
In the next set of analyses, we compared the background and perioperative outcomes between the two MIS approaches: laparoscopic and robotic.
Patient and tumor background
Background data were compared between the laparoscopic and robotic groups [Table 4]. Before PSM (59 laparoscopic and 21 robotic cases), compared to the laparoscopic group, the robotic group was significantly associated with a lower rate of cirrhosis, smaller tumor size, lower AFP level, as well as a higher rate of repeat hepatectomy with higher numbers of previous hepatectomy and previous open liver resection. Further, the tumor number tended to be smaller in the robotic group without statistical significance. After 1:1 PSM (16:16), all studied variables were comparable between the laparoscopic and robotic groups.
Comparison of background data between laparoscopic and robotic cohorts undergoing segmentectomy for HCC before and after PSM
| Before PSM | After PSM | |||||
| Laparoscopic (n = 59) | Robotic (n = 21) | P | Laparoscopic (n = 16) | Robotic (n = 16) | P | |
| Age, years | 70 (36-86) | 72 (48-81) | 0.447 | 71 (57-86) | 72 (48-79) | 0.895 |
| Sex, male/female | 47/12 | 19/2 | 0.263 | 14/2 | 14/2 | 1.000 |
| BMI, kg/m2 | 23.5 (18.0-36.3) | 23.0 (17.9-30.3) | 0.874 | 24.0 (20.2-31.2) | 23.1 (17.9-30.3) | 0.836 |
| ASA score, I or II/≥ III | 58/1 | 19/2 | 0.105 | 16/0 | 16/0 | 1.000 |
| Child-Pugh class, A/B/C | 58/1/0 | 21/0/0 | 0.548 | 16/0/0 | 16/0/0 | 1.000 |
| Cirrhosis (pathologic), n (%) | 30/29 | 3/18 | 0.004 | 3/13 | 3/13 | 1.000 |
| ICGR15, % ≥ 13.0%, n (%) | 11.3 (3.4-68.3) 21 (37.5) | 11.2 (2.2-30.8) 8 (40.0) | 0.396 0.843 | 10.3 (4.2-68.3) 3 (21.4) | 11.2 (2.2-30.8) 6 (37.5) | 0.868 0.338 |
| Tumor number Single/Multiple | 1 (1-3) 51/8 | 1 (1-4) 15/6 | 0.095 0.120 | 1 (1-2) 14/2 | 1 (1-4) 14/2 | 0.978 1.000 |
| Tumor size, cm ≥ 3.0 cm, n (%) | 3.0 (0.7-11.0) 34 (57.6) | 2.1 (1.2-6.0) 6 (28.6) | 0.017 0.022 | 2.6 (1.5-10.8) 6 (37.5) | 2.5 (1.2-6.1) 5 (31.3) | 0.354 0.710 |
| Etiology, viral/non-viral | 37/22 | 12/9 | 0.653 | 9/7 | 10/5 | 0.719 |
| Posterosuperior lesion, n (%) | 38 (64.4) | 10 (47.6) | 0.178 | 9 (56.3) | 7 (43.8) | 0.480 |
| AFP, ng/mL | 7.6 (2.0-15,412.0) | 3.5 (1.0-253.2) | 0.001 | 5.0 (2.3-7,194.0) | 4.6 (1.0-253.2) | 0.274 |
| DCP, mAU/mL | 61 (10-11,256) | 33 (13-30,899) | 0.220 | 46 (16-4,923) | 34 (17-20,843) | 0.509 |
| Pathological stage, I/II/≥ III | 12/38/9 | 4/14/3 | 0.983 | 3/11/2 | 3/12/1 | 0.828 |
| Previous hepatectomy Yes, n (%) Number, 0/1/≥ 2 Including open Hx, n (%) | 8 (13.6) 51/8/0 2 (3.4) | 7 (33.3) 14/4/3 4 (19.1) | 0.046 0.009 0.038 | 4 (25.0) 12/4/0 1 (6.3) | 3 (18.8) 13/2/1 2 (12.5) | 0.669 0.426 0.542 |
Perioperative outcomes
Perioperative outcomes are shown in Table 5. Before PSM, laparoscopic and robotic groups had comparable outcomes, except for the significantly higher maximum AST level (893 vs. 537 IU/L; P = 0.041) in the robotic group. After PSM, both groups had comparable perioperative outcomes.
Comparison of perioperative outcomes between laparoscopic and robotic cohorts undergoing segmentectomy for HCC before and after PSM
| Before PSM | After PSM | |||||
| Laparoscopic (n = 59) | Robotic (n = 21) | P | Laparoscopic (n = 16) | Robotic (n = 16) | P | |
| IWATE criteria, level Intermediate Advanced Expert | 16 34 9 | 9 11 1 | 0.264 | 4 10 7 | 7 9 0 | 0.238 |
| Operative time, min | 638 (525-773) | 676 (544-1,009) | 0.199 | 637 (536-705) | 652 (523-882) | 0.598 |
| Parenchymal dissection time, min | 236 (175-310) | 273 (193-374) | 0.260 | 207 (175-283) | 241 (171-380) | 0.477 |
| Blood loss, g | 220 (141-486) | 190 (90-729) | 0.848 | 168 (116-250) | 154 (74-683) | 0.910 |
| Transfusion, n (%) | 10 (17.0) | 4 (19.1) | 0.828 | 0 (0) | 2 (12.5) | 0.144 |
| Pringle maneuver, n (%) | 8 (13.6) | 6 (28.6) | 0.120 | 1 (6.3) | 3 (18.8) | 0.285 |
| Open conversion, n (%) | 0 (0) | 1 (4.8) | 0.092 | 0 (0) | 0 (0) | 1.000 |
| Max-TB, mg/dL | 1.5 (1.2-1.9) | 1.5 (1.5-2.0) | 0.254 | 1.4 (1.1-1.5) | 1.7 (1.4-2.1) | 0.061 |
| Max-AST, IU/L | 537 (316-944) | 893 (448-1,885) | 0.041 | 505 (315-886) | 844 (434-1,288) | 0.163 |
| Min-PT, % | 64 (59-73) | 59 (50-72) | 0.118 | 62 (59-75) | 59 (47-73) | 0.282 |
| R0 resection, % | 100 | 100 | 1.000 | 100 | 100 | 1.000 |
| Morbidity ≤ 90 days, n (%) Overall Major (≥ C-D IIIa) Bile leak/collection | 24 (40.7) 5 (8.5) 3 (5.1) | 10 (47.6) 4 (19.1) 0 (0) | 0.581 0.188 0.292 | 4 (25.0) 2 (12.5) 1 (6.3) | 6 (37.5) 3 (18.8) 0 (0) | 0.446 0.626 0.310 |
| Mortality, n (%) ≤ 30 days ≤ 90 days | 0 (0) 1 (1.7) | 0 (0) 0 (0) | 1.000 0.548 | 0 (0) 1 (6.3) | 0 (0) 0 (0) | 1.000 0.310 |
| Length of hospital stay, days | 15 (12-20) | 16 (14-20) | 0.673 | 13 (11-17) | 15 (13-20) | 0.199 |
Long-term outcomes after IA-Seg for newly developed HCC
In the next set of analyses, we studied long-term outcomes after IA-Seg in 128 patients with newly developed HCC, who underwent respective 63 open and 65 MIS (51 laparoscopic and 14 robotic) IA-Seg, and compared OS and RFS between the open and MIS approaches and between the laparoscopic and robotic approaches.
Comparison between the open and MIS approaches
Patient and tumor background
Sixty-three patients undergoing open IA-Seg and 65 patients undergoing minimally invasive IA-Seg were compared in terms of patient and tumor characteristics [Table 6]. Before PSM (63:65), compared to the open approach, MIS was significantly associated with the lower ASA class, lower ICGR 15, smaller tumor number, more favorable tumor stages, and lower R0 resection rate. DCP tended to be lower in MIS, but the difference was insignificant. After 1:1 PSM to reduce biases (36:36), all studied variables were comparable between the open and MIS groups.
Comparison of background data between open and MIS cohorts undergoing segmentectomy for newly developed HCC before and after PSM
| Before PSM | After PSM | |||||
| Open (n = 63) | MIS (n = 65) | P | Open (n = 36) | MIS (n = 36) | P | |
| Age, year | 73 (43-91) | 70 (36-86) | 0.105 | 73 (43-91) | 72 (36-85) | 0.718 |
| Sex, male/female, n | 49/14 | 52/13 | 0.758 | 28/8 | 29/7 | 0.772 |
| ASA score, I or II/≥ III, n | 45/18 | 63/2 | < 0.0001 | 34/2 | 34/2 | 1.000 |
| Child-Pugh class, A/B/C, n | 58/5/0 | 64/1/0 | 0.087 | 34/2/0 | 35/1/0 | 0.555 |
| Cirrhosis (pathologic), n (%) | 27 (42.9) | 25 (38.5) | 0.613 | 15 (41.7) | 15 (41.7) | 1.000 |
| ICGR15, % ≥ 13%, n (%) | 15.0 (0.6-41.0) 39 (62.9) | 11.1 (2.2-39.4) 23 (36.5) | 0.005 0.003 | 14.1 (0.6-41.0) 16 (44.4) | 13.9 (2.2-39.4) 15 (41.7) | 0.437 0.812 |
| Tumor number Single/Multiple, n | 1 (1-23) 43/20 | 1 (1-4) 54 /11 | 0.046 0.049 | 1 (1-5) 27/9 | 1 (1-4) 28/8 | 0.838 0.781 |
| Tumor size ≥ 3cm, n (%) | 3.5 (1.2-17.0) 43 (63.5) | 3.1 (0.7-11) 39 (60.0) | 0.154 0.685 | 3.6 (1.2-17.0) 24 (66.7) | 3.3 (0.7-11.0) 23 (63.9) | 0.269 0.805 |
| Anterolateral/Posterosuperior, n | 22/41 | 25/40 | 0.678 | 14/22 | 13/23 | 0.808 |
| AFP, ng/mL | 10.9 (2.3-28,180.0) | 6.5 (1.4-15,412.0) | 0.360 | 9.8 (2.5-9,439.5) | 10.8 (1.4-15,412.0) | 0.973 |
| DCP, mAU/mL | 116 (10-123,650) | 50 (10-20,843) | 0.050 | 123 (10-123,650) | 50 (10-20,843) | 0.068 |
| Pathological stage, n I/II/III/IVA/IVB I or II/≥ III | 6/35/19/1/2 41/22 | 8/46/11/0/0 54/11 | 0.142 0.019 | 2/24/8/1/1 26/10 | 3/23/10/0/0 26/10 | 0.655 1.000 |
| Differentiation, n (%) Well Moderate Poor or sarcomatous Combined | 3 (4.8) 56 (88.9) 3 (4.8) 1 (1.6) | 4 (6.2) 57 (87.7) 3 (4.6) 1 (1.5) | 0.989 | 1 (2.8) 31 (86.1) 3 (8.3) 1 (2.8) | 0 (0) 35 (97.2) 0 (0) 1 (2.8) | 0.236 |
| R0 resection, n (%) | 60 (95.2) | 65 (100) | 0.038 | 34 (94.4) | 36 (100) | 0.152 |
Long-term outcomes
Before PSM, compared to open surgery (n = 63), MIS (n = 65) had comparable or potentially more favorable OS (Figure 5A; P = 0.020; 5-year rate: 86.0% vs. 61.6%) and RFS (Figure 5B; P = 0.047; 5-year rate: 49.3% vs. 34.5%) in. After PSM (36:36), both OS (Figure 5C; P = 0.063) and RFS (Figure 5D; P = 0.069) were comparable between open and minimally invasive IA-Seg.
Comparison between the laparoscopic and robotic approaches
Patient and tumor background
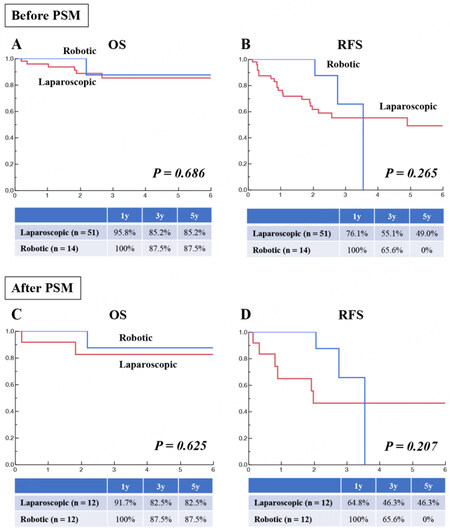
Fifty-one patients undergoing laparoscopic IA-Seg and 14 patients undergoing robotic IA-Seg were compared in terms of patient and tumor characteristics [Table 7]. Before PSM (51:14), compared to the laparoscopic approach, the robotic approach was significantly associated with the lower rate of cirrhosis and had an insignificant tendency to the lower AFP level. After 1:1 PSM to reduce biases (12:12), all studied variables were comparable between the laparoscopic and robotic groups.
Comparison of background between laparoscopic and robotic cohorts undergoing segmentectomy for newly developed HCC before and after PSM
| Before PSM | After PSM | |||||
| Laparoscopic (n = 51) | Robotic (n = 14) | P | Laparoscopic (n = 12) | Robotic (n = 12) | P | |
| Age, year | 69 (36-86) | 72 (48-79) | 0.805 | 69 (57-86) | 72 (48-79) | 0.623 |
| Sex, male/female, n | 40/11 | 12/2 | 0.546 | 11/1 | 11/1 | 1.000 |
| ASA score, I or II/≥ III, n | 50/1 | 13/1 | 0.320 | 12/0 | 11/1 | 0.307 |
| Child-Pugh class, A/B/C, n | 50/1/0 | 14/0/0 | 0.598 | 12/0/0 | 12/0/0 | 1.000 |
| Cirrhosis (pathologic), n (%) | 24 (47.1) | 1 (7.1) | 0.003 | 11/1 | 11/1 | 1.000 |
| ICGR15, % ≥ 13%, n (%) | 11.6 (3.4-39.4) 19 (38.8) | 10.7 (2.2-17.5) 4 (28.6) | 0.261 0.484 | 12.2 (4.2-20.9) 2 (18.2) | 9.1 (2.2-16.0) 3 (25.0) | 0.356 |
| Tumor number Single/Multiple, n | 1 (1-3) 43/8 | 1 (1-4) 11/3 | 0.582 0.612 | 1 (1-2) 10/2 | 1 (1-4) 10/2 | 0.929 1.000 |
| Tumor size ≥ 3cm, n (%) | 3.2 (0.7-11.0) 33 (64.7) | 2.6 (1.5-6.0) 6 (42.9) | 0.227 0.143 | 2.9 (1.5-10.8) 6 (50.0) | 2.8 (2.1-6.0) 6 (50.0) | 0.885 1.000 |
| Anterolateral/Posterosuperior, n | 18/33 | 7/7 | 0.321 | 5/7 | 6/6 | 0.682 |
| IWATE criteria, Level Advanced or Expert, n (%) | 38 (74.5) | 9 (64.3) | 0.449 | 10 (83.3) | 8 (66.7) | 0.346 |
| AFP, ng/mL | 7.6 (2.0-15,412.0) | 4.6 (1.4-253.2) | 0.059 | 5.6 (2.3-7,194.0) | 4.6 (1.4-253.2) | 0.248 |
| DCP, mAU/mL | 65 (10-11,256) | 33 (17-20,843) | 0.194 | 37 (16-4,923) | 33 (21-20,843) | 0.840 |
| Pathological stage, n I/II/III/IVA/IVB I or II/≥ III | 8/34/9/0/0 42/8 | 0/12/2/0/0 12/2 | 0.243 0.766 | 2/8/2/0/0 10/2 | 0/10/2/0/0 10/2 | 0.329 1.000 |
| Differentiation, n (%) Well Moderate Poor or sarcomatous Combined | 4 (7.8) 43 (84.3) 3 (5.9) 1 (2.0) | 0 (0) 14 (100) 0 (0) 0 (0) | 0.475 | 2 (16.7) 9 (75.0) 1 (8.3) 0 (0) | 0 (0) 12 (100) 0 (0) 0 (0) | 0.180 |
Long-term outcomes
Comparison between the laparoscopic (n = 51) and robotic (n = 14) approaches before PSM showed comparable OS [Figure 6A] and RFS [Figure 6B]. Comparison between the two groups after PSM (12:12) also showed comparable OS [Figure 6C] and RFS [Figure 6D].
DISCUSSION
In this study, we described our surgical techniques of minimally invasive IA-Seg and examined perioperative and long-term outcomes in HCC patients undergoing open, laparoscopic and robotic IA-Seg. To the best of our knowledge, this is the first PSM-based comparative study limited to IA-Seg for HCC, between open surgery and MIS, or between the laparoscopic and robotic approaches. Furthermore, this study was conducted on a confined HCC cohort, where IA-Seg was performed consistently using the extrahepatic Glissonian approach and HV root-at first parenchymal dissection, irrespective of MIS or open surgery, for tumors at posterosuperior “difficult” segments in over 60% of cases, by surgeons with the expertise of both open and minimally invasive IA-Seg. Such restricted study settings may have lessened the effects of biases such as disease, tumor location, detailed types of resection, surgical techniques, and surgeons’ experience, which were latent in many previous comparative studies[15,24-29].
In the current study, we first audited perioperative outcomes of 80 minimally invasive IA-Seg for HCC and compared them to those in the 77 open counterparts. A PSM (46:46)-based comparison showed significantly less blood loss, a lower transfusion rate, a lower postoperative TB level, and shorter LOS in the MIS group than in the open group, while a significantly higher rate of Pringle maneuver application and a higher AST level were observed in the MIS group. Next, we compared perioperative outcomes between laparoscopic and robotic IA-Seg, and found comparable outcomes between the cohorts, either before or after PSM. Finally, we studied long-term outcomes after IA-Seg for newly developed HCC. PSM-based analyses showed comparable long-term outcomes, either between the open surgery and MIS or between the laparoscopic and robotic approaches, although the small sample size in each group is a critical limitation that precludes definite conclusions.
Many previous studies comparing open and MIS hepatectomy have shown less blood loss, decreased morbidity, and shorter hospital stay in the latter[15,24-29]. Further, laparoscopic and robotic liver resections were shown to have comparable perioperative outcomes in many studies[15,24-26]. The results of the current study are in line with those of these previous studies, although the cohort setting was different. The higher rate of Pringle maneuver application and the (probably associated) higher postoperative AST level in MIS may be related to surgeons’ preference to maintain a bloodless operative field in the magnified view during MIS. On the other hand, long-term outcomes were comparable between open and MIS IA-Seg, and between the laparoscopic and robotic approaches. These results were also consistent with those of previous studies, though background settings in these studies were different from ours[15,24,29].
In view of our results showing more favorable perioperative outcomes in MIS than in open IA-Seg and comparable long-term outcomes, it is not unreasonable to suggest that MIS would be the first choice of the IA-Seg approach to HCC, at least by the experts’ hands. On the other hand, despite the expected functional merits of robotics including instrument articulation, tremor filtering and stabilized visual field, the robotic approach was unable to show advantages in surgical outcomes over the laparoscopic approach to IA-Seg. However, the robotic platform may still have potential advantages in perioperative outcomes in this complex anatomic resection, such as less blood loss, decreased open conversion rate and decreased morbidity, as suggested in the other study settings[30-32]. Larger studies are warranted to investigate the potentially significant differences in outcomes between laparoscopic and robotic IA-Seg.
We consider that minimally invasive IA-Seg is a demanding procedure for the following reasons. First, the methods to determine the anatomically accurate liver segments during this type of hepatectomy are not well standardized. The reduced range of instrumental motion during the Glissonian approach to the segment-feeding GPs is still a critical obstacle during IA-Seg, though robotics can overcome such limitations. Furthermore, Makuuchi’s dyeing method[1] is much less applicable in MIS than in open surgery because of the technical difficulty and lack of suitable laparoscopic or robotic instruments for needle puncture. Second, as major or minor intersegmental hepatic veins usually run along the segmental borders, these veins need to be exposed on the intersegmental planes during IA-Seg, which is still a demanding technique. Third, accurate resection of the optimal amount of parenchyma of the target liver segment requires expertise during MIS.
Nonetheless, from our surgical results, we believe that standardization for IA-Seg is possible for any liver segments not only in open surgery but also in MIS, by using the extrahepatic Glissonian approach and HV root-at first cranial-to-caudal parenchymal dissection[9,12,13,20,21]. These techniques for IA-Seg, which were originally developed in open surgery[9,12,21], have been translated into MIS with acceptable safety, feasibility and curability, as shown by the perioperative and long-term outcomes in this study. Moreover, robotics, which was the most recently developed platform in our series, was technically applicable to IA-Seg with comparable surgical outcomes with the laparoscopic approach.
Intraoperative visualization of an isolated target liver segment is a critical step during IA-Seg. In open surgery, Makuuchi’s US-guided transportal dye injection method[1] and a method using US-guided finger compression of the feeding pedicles of the target segments[33] are useful. In contrast, in the current setting of MIS, direct injection of staining dye into a segmental portal vein branch by intracorporeal US-guided needle puncture is technically challenging[11]. Therefore, currently, for technical simplicity and reliability, the ‘negative-staining method’ using ICG would be the first choice to determine the target segment during laparoscopic and robotic IA-Seg[9,15]. For accurate application of the “negative-staining method”, the target segmental GPs need to be selectively clamped. For this purpose, the extrahepatic Glissonian approach is more useful and accurate than the intrahepatic Glissonian approach because, in the former, the target segment is identified and clearly visualized as a negatively stained area, before any parenchymal dissection is started [Figure 3D].
There are several limitations to this study. First, the sample size in each cohort is small, which precludes definite conclusions. Second, this is a retrospective, observational, single-center study, though PSM was conducted to reduce biases. Third, long-term outcomes should be carefully interpreted because of the small sample size, small number of matched variables in PSM and biases related to the surgery era, which affected several factors, including selection of approach, learning curve of surgical techniques, development of drugs for eradication of hepatitis C virus and introduction of new anti-HCC drugs.
In conclusion, although minimally invasive IA-Seg is technically demanding, it could be standardized by the extrahepatic Glissonian approach and HV root-at first parenchymal dissection. This MIS procedure for HCC is safe, feasible and oncologically acceptable, with perioperative outcomes mostly superior to those in open surgery and with comparable long-term outcomes. The laparoscopic or robotic approach could be a reliable choice for IA-Seg in selected surgical HCC patients, at least by expert hands. Further larger studies are needed to investigate the potential advantages of MIS over open IA-Seg in long-term outcomes, as well as those of the robotic over laparoscopic IA-Seg in surgical outcomes.
DECLARATIONS
AcknowledgementsThe authors thank Masayuki Kojima, Satoshi Mii, Yuichiro Uchida, Hideaki Iwama, Takeshi Takahara, Yoshinao Tanahashi, Akira Yasuda, Sanae Nakajima, Gozo Kiguchi, Junichi Yoshikawa, and Koichi Suda for their contributions to surgery and data acquisition.
Authors’ contributionsMade substantial contributions to the conception and design of the study and performed data analysis and interpretation: Kato Y, Sugioka A, Uyama I
Performed data acquisition: Kato Y
Provided technical support: Sugioka A, Uyama I
Availability of data and materialsThe datasets are not available for public access due to patient privacy concerns but are available from the corresponding author upon reasonable request.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateThis study was conducted in accordance with the Declaration of Helsinki (2000) and was approved by the institutional research board (approval number: HM19-064). Informed consent was obtained from all patients.
Consent for publicationWritten informed consent for publication was obtained from all patients.
Copyright© The Author(s) 2023.
REFERENCES
1. Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Obstet Gynecol 1985;161:346-50.
2. Hasegawa K, Kokudo N, Imamura H, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg 2005;242:252-9.
3. Shindoh J, Makuuchi M, Matsuyama Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol 2016;64:594-600.
4. Takasaki K, Kobayashi S, Tanaka S, Saito A, Yamamoto M, Hanyu F. Highly anatomically systematized hepatic resection with Glissonean sheath code transection at the hepatic hilus. Int Surg 1990;75:73-7.
5. Liau KH, Blumgart LH, DeMatteo RP. Segment-oriented approach to liver resection. Surg Clin North Am 2004;84:543-61.
6. Kishi Y, Hasegawa K, Kaneko J, et al. Resection of segment VIII for hepatocellular carcinoma. Br J Surg 2012;99:1105-12.
7. Mazziotti A, Maeda A, Ercolani G, Cescon M, Grazi GL, Pierangeli F. Isolated resection of segment 8 for liver tumors: a new approach for anatomical segmentectomy. Arch Surg 2000;135:1224-9.
8. Ome Y, Honda G, Doi M, Muto J, Seyama Y. Laparoscopic anatomic liver resection of segment 8 using intrahepatic glissonean approach. J Am Coll Surg 2020;230:e13-e20.
9. Kato Y, Sugioka A, Kojima M, et al. Laparoscopic isolated liver segmentectomy 8 for malignant tumors:techniques and comparison of surgical results with the open approach using a propensity score-matched study. Langenbecks Arch Surg 2022;407:2881-92.
10. Berardi G, Igarashi K, Li CJ, et al. Parenchymal sparing anatomical liver resections with full laparoscopic approach: description of technique and short-term results. Ann Surg 2021;273:785-91.
11. Otsuka Y, Matsumoto Y, Ito Y, et al. Intraoperative guidance using ICG fluorescence imaging system for safe and precise laparoscopic liver resection. Minerva Surg 2021;76:211-9.
12. Sugioka A, Kato Y, Tanahashi Y, et al. Standardization of anatomic liver resection based on Laennec’s capsule. Surg Gastroenterol Oncol 2020;25:57-66.
13. Kato Y, Sugioka A, Uyama I. Robotic liver resection for hepatocellular carcinoma:a focus on anatomic resection. Hepatoma Res 2021;7:10.
14. Lee JH, Han DH, Jang DS, Choi GH, Choi JS. Robotic extrahepatic Glissonean pedicle approach for anatomic liver resection in the right liver: techniques and perioperative outcomes. Surg Endosc 2016;30:3882-8.
15. Kato Y, Sugioka A, Kojima M, et al. Initial experience with robotic liver resection: audit of 120 consecutive cases at a single center and comparison with open and laparoscopic approaches. J Hepatobiliary Pancreat Sci 2023;30:72-90.
16. Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol 1993;9:298-304.
17. Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer. Jan J Surg 1989;19:98-129.
18. Strasberg SM, Belghetti J, Clavien PA, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000;2:333-9.
19. Couinaud C. Le foie: etudes anatomiques et chirurgicales. Paris: Masson; 1957.
20. Sugioka A, Kato Y, Tanahashi Y. Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec’s capsule: proposal of a novel comprehensive surgical anatomy of the liver. J Hepatobiliary Pancreat Sci 2017;24:17-23.
21. Kato Y, Sugioka A, Tanahashi Y, et al. Standardization of isolated caudate lobectomy by extrahepatic Glissonean pedicle isolation and HV root-at first one-way resection based on Laennec’s capsule:open and laparoscopic approaches. Surg Gastroenterol Oncol 2020;25:89-92.
22. Wakabayashi G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr 2016;5:281-9.
23. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13.
24. Takahara T, Wakabayashi G, Beppu T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching:a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci 2015;22:721-7.
25. Tozzi F, Berardi G, Vierstraete M, et al. Laparoscopic versus open approach for formal right and left hepatectomy: a propensity score matching analysis. World J Surg 2018;42:2627-34.
26. Untereiner X, Cagniet A, Memeo R, et al. Laparoscopic hepatectomy versus open hepatectomy for the management of hepatocellular carcinoma: a comparative study using a propensity score matching. World J Surg 2019;43:615-25.
27. Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549-55.
28. Fruscione M, Pickens R, Baker EH, et al. Robotic-assisted versus laparoscopic major liver resection:analysis of outcomes from a single center. HPB 2019;21:906-11.
29. Lim C, Salloum C, Tudisco A, et al. Short- and long-term outcomes after robotic and laparoscopic liver resection for malignancies: a propensity score-matched study. World J Surg 2019;43:1594-1603.
30. Kadam P, Sutcliffe RP, Scatton O, et al. An international multicenter propensity-score matched and coarsened-exact matched analysis comparing robotic versus laparoscopic partial liver resections of the anterolateral segments. J Hepatobiliary Pancreat Sci 2022;29:843-54.
31. Sucandy I, Rayman S, Lai EC, et al. Robotic versus laparoscopic left and extended left hepatectomy: an international multicenter study propensity score-matched analysis. Ann Surg Oncol 2022;29:8398-406.
32. Chong CC, Fuks D, Lee KF, et al. Propensity score-matched analysis comparing robotic and laparoscopic right and extended right hepatectomy. JAMA Surg 2022;157:436-44.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Kato Y, Sugioka A, Uyama I. Minimally invasive isolated anatomic liver segmentectomy for hepatocellular carcinoma using extrahepatic Glissonian approach: surgical techniques and outcomes. Mini-invasive Surg 2023;7:11. http://dx.doi.org/10.20517/2574-1225.2022.110
AMA Style
Kato Y, Sugioka A, Uyama I. Minimally invasive isolated anatomic liver segmentectomy for hepatocellular carcinoma using extrahepatic Glissonian approach: surgical techniques and outcomes. Mini-invasive Surgery. 2023; 7: 11. http://dx.doi.org/10.20517/2574-1225.2022.110
Chicago/Turabian Style
Kato, Yutaro, Atsushi Sugioka, Ichiro Uyama. 2023. "Minimally invasive isolated anatomic liver segmentectomy for hepatocellular carcinoma using extrahepatic Glissonian approach: surgical techniques and outcomes" Mini-invasive Surgery. 7: 11. http://dx.doi.org/10.20517/2574-1225.2022.110
ACS Style
Kato, Y.; Sugioka A.; Uyama I. Minimally invasive isolated anatomic liver segmentectomy for hepatocellular carcinoma using extrahepatic Glissonian approach: surgical techniques and outcomes. Mini-invasive. Surg. 2023, 7, 11. http://dx.doi.org/10.20517/2574-1225.2022.110
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 7 clicks
Cite This Article 7 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.